 Short Answer Type
Short Answer TypeA reaction is of second order with respect to a reactant. How is its rate affected if the concentration of the reactant is (i) doubled (ii) reduced to half?
Express the relation among cell constant, the resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity?
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9S cm2mol-1. Calculate the conductivity of the conductivity of this solution.
Explain the role of each of the following:
(i) NaCN in the extraction of silver
(ii) SiO2 in the extraction of copper
(i) The roasted ore of gold is leached with a solution of sodium cyanide in the presence of oxygen for many days. The role of NaCN in this process is to dissolve the gold to form an aurocyanide complex, from which the metal is obtained by displacement.
4Au + 8NaCN + 2H2O + O2 ---> 4 Na [Au(CN)2] + 4KOH
2Na[Au(CN)2] + Zn ---> Na2[Zn(CN)4] + 2Au
(ii) Copper matte contains Cu2S and FeS. In the blast furnace, copper matte is added with powdered coke and silica. The oxidation of ore takes place in this process. As a result, cuprous oxide and ferrous oxide are produced. The role of silica in this process is to remove the iron oxide obtained as ‘slag’. FeO combines with silica (flux) to form iron silicate, FeSiO3(slag).
FeO + SiO2 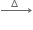 FeSiO2
FeSiO2
Flux slag
Explain the following facts giving appropriate reason in each case:
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) All the bonds in SF4 are not equivalent.
