Short Answer Type
Short Answer TypeA reaction is of second order with respect to a reactant. How is its rate affected if the concentration of the reactant is (i) doubled (ii) reduced to half?
Express the relation among cell constant, the resistance of the solution in the cell and conductivity of the solution. How is molar conductivity of a solution related to its conductivity?
The molar conductivity of a 1.5 M solution of an electrolyte is found to be 138.9S cm2mol-1. Calculate the conductivity of the conductivity of this solution.
Explain the role of each of the following:
(i) NaCN in the extraction of silver
(ii) SiO2 in the extraction of copper
Explain the following facts giving appropriate reason in each case:
(i) NF3 is an exothermic compound whereas NCl3 is not.
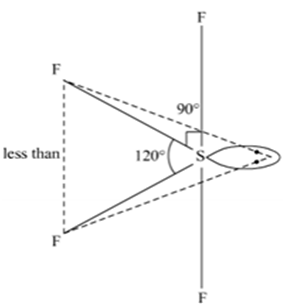
(ii) All the bonds in SF4 are not equivalent.
(i) As we move down the group 17, the size of the atom increases from fluorine to chlorine. The instability of NCl3 is due to the weak NCl bond. This is due to the large difference in the size of nitrogen and chlorine atoms. On the other hand, atoms of both nitrogen (75 pm) and fluorine (72 pm) are small sized. Thus, bonding in NF3 is quite strong and it is an exothermic compound.
(ii)SF4 has four bonded atoms and one lone pair so SF4 has sp3d hydridisation and thus have trigonal bipyramid structure in which one axial position is occupied by a lone pair of electrons. This lone pair finds a position that minimizes the number of 900 repulsion it has with bonding electron pairs. This results in two types of angles.
Equatorial bond angle F - S - F° (LP-BP repulsion >BP – BP repulsion)
Axial bond angle F - S - F<90°