 Short Answer Type
Short Answer TypeUsing IUPAC norms write the formulate for the following coordination compounds:
(i) Hexaamminecobalt (III) chloride
(ii) Potassium tetrachloridonickelate (II)
Arrange the following in increasing order of their basic strength:
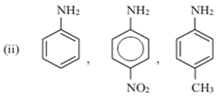
(i) C6H5 – NH2, C6H5 – CH2 – NH2, C6H5 – NH – CH3
Why does a solution containing non-volatile solute have higher boiling point than the pure solvent? Why is elevation of boiling point a colligative property?
(i) What is the principle behind the zone refining of metals?
(ii) What is the role of silica in the extraction of copper?
(iii) How is 'cast iron' different from 'pig iron'?
(i) The technique of zone refining is based on the principle that the impurities are more soluble in the molten state of metal than in the solid state. Silicon , boron gallium , indium etc. can purify by this process.
(ii) Ores of copper contain iron oxide as an impurity. Silica reacts with iron oxide and forms iron silicate, which is removed as slag.
FeO + SiO2 -------> FeSiO3
Iron oxide silica iron silicate (slag)
(iii) The iron obtained from the blast furnace is known as pig iron. It contains around 4% carbon and many impurities, such as S, P, Si, Mn, in smaller amounts.
Cast iron is obtained by melting pig iron and coke using a hot air blast. It contains a lower amount of carbon (about 3%) than pig iron. Unlike pig iron, cast iron is extremely hard and brittle.
