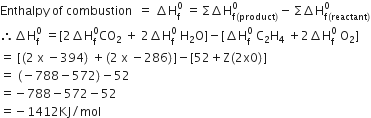Short Answer Type
Short Answer Type(i) How many sodium ions and chloride ions are present in a unit cell of sodium chloride ?
(ii) What is the co-ordination number of sodium and chloride ions in sodium chloride crystals ?
Give a reason for each of the following :
(i) Specific conductance decreases with dilution whereas equivalent conductance increases with dilution.
(ii) Lead is precipitated as PbS while zinc is not precipitated when H2S gas is passed through an acidic solution of lead nitrate and zinc nitrate.
(i) Enthalpy change and entropy change of a chemical reaction at 25°C are 177.0 KJ and 160.4 JK-1 respectively. Calculate free energy change of the reaction and predict whether the reaction is spontaneous or nonspontaneous.
(ii) 2 moles of an ideal gas at 27°C expand reversibly from 2 litres to 20 litres. Find the entropy change for the reaction.
(iii) State the second law of thermodynamics in terms of entropy of the universe.
C2H4 + 202→2 C02 + 2 H20
Given ΔH f0 C2H4 = 52 KJ/mole
ΔH f0 C02 = - 394 KJ/mole
ΔHf0 H20 = -286 KJ/mole