 Multiple Choice Questions
Multiple Choice QuestionsWhich one ofthe following is a correct set?
H2O, sp3, angular
H2O, sp2, linear
NH4+, dsp2,square planar
CH4, dsp2, tetrahedral
Which one of the following represents the graph between log p (on Y-axis) and 1/T on X-axis)? (p = vapour pressure of a liquid, T = absolute temperature).
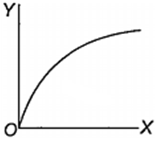
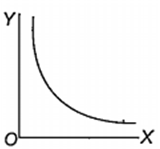
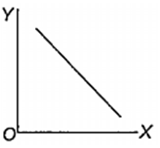
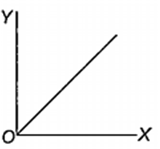
What is the volume (in litres) of oxygen required at STP to completely convert 1.5 mol of sulphur into sulphur dioxide?
11.2
22.4
33.6
44.8
If a gas contains only three molecules that move with velocities of 100, 200, 500 ms-1. What is the rms velocity of that gas in ms-1?
100
100√30
100√10
800/3
When 10 g of methane is completely burnt in oxygen, the heat evolved is 560 kJ. What is the heat of combustion (in kJ mol-1) of methane?
-1120
-968
-896
-560
If the ionic product of water (Kw) is 1.96 x 10-14 at 35°C, what is its value at 10°C?
1.96 × 10-14
3.92 × 10-14
2.95 × 10-14
1.96 × 10-13
If the electron of a hydrogen atom is present in the first orbit, the total energy of the electron is
If the wavelength of an electromagnetic radiation is 2000 Å, what is its energy in ergs?
9.94 × 10-12
9.94 × 10-19
4.97 × 10-12
4.97 × 10-19
If the bond length and dipole moment of a diatomic molecule are 1.25 A and 1.0 D respectively, what is the percent ionic character of the bond ?
10.66
12.33
16.66
19.33
Which one of the following reactions occur at the anode, in the Castner process of extracting sodium metal ?
H2 → 2H+ + 2e-
2Cl- → Cl2 + 2e-
4OH- → 2H2O + O2 + 4e-
Na+ + e- → Na