 Multiple Choice Questions
Multiple Choice QuestionsOne mole of magnesium nitride on the reaction with an excess of water gives
one mole of ammonia
two moles of nitric acid
Two mole of ammonia
Two mole of ammonia
Which one of the following ores is best concentrated by froth – floatation method?
Magnetite
Malachite
Galena
Galena
Beryllium and aluminium exhibit many properties which are similar. But the two elements differ in
exhibiting maximum covalency in compound
exhibiting amphoteric nature in their oxides
forming covalent halides
forming covalent halides
Aluminium chloride exists as a dimer, Al2Cl6 in the solid state as well as in solution of non-polar solvents such as benzene. When dissolved in water, it gives
Al3+ + 3Cl-
Al2O3 + 6HCl
[Al(OH)6] 3-
[Al(OH)6] 3-
The soldiers of Napolean army while at Alps during freezing winter suffered a serious problem as regards to the tin buttons of their uniforms. White metallic tin buttons got converted to grey powder. This transformation is related to
an interaction with nitrogen of the air at very low temperatures
an interaction with water vapour contained in the humid air
a change in the partial pressure of oxygen in the air
a change in the partial pressure of oxygen in the air
The 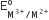 values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
values for Cr, Mn, Fe and Co are – 0.41, +1.57, + 0.77 and +1.97 V respectively. For which one of these metals the change in oxidation state form +2 to +3 is easiest?
Cr
Co
Fe
Fe
Excess of KI reacts with CuSO4 solution and then Na2S2O3 solution is added to it. Which of the statements is incorrect for this reaction?
Cu2I2 is reduced
Evolved I2 is reduced
Na2S2O3 is oxidized
Na2S2O3 is oxidized
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by CN- ion towards metal species is
a, b
a, b, c
c, a
c, a
The coordination number of central metal atom in a complex is determined by
The number of ligands around a metal ion bonded by sigma bonds
The number of only anionic ligands bonded to the metal ion
The number of ligands around a metal ion bonded by sigma and pi- bonds both
The number of ligands around a metal ion bonded by sigma and pi- bonds both
Which one of the following complexes in an outer orbital complex?
[Fe(CN)6]4-
[Ni(NH3)6]2+
[Co(NH3)6]3+
[Co(NH3)6]3+
B.
[Ni(NH3)6]2+

