 Multiple Choice Questions
Multiple Choice QuestionsWhat type of crystal defect is indicated in the diagram below?
Frenkel defect
Frenkel and Schottky defects
Interstitial defect
Interstitial defect
D.
Interstitial defect
When the equal number of cations and anions are missing. Then it is the case of Schottky defect.
In hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
generate heat
remove adsorbed oxygen from electrode surfaces
produce high purity water
produce high purity water
In first order reaction, the concentration of the reactant decreases from 0.8 M to 0.4 M in 15 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M is
30 minutes
60 minutes
7.5 minutes
7.5 minutes
The rate equation for the reaction 2A + B → C is found to be: rate k[A][B]. The correct statement in relation to this reaction is that the
unit of K must be s-1
values of k is independent of the initial concentration of A and B
rate of formation of C is twice the rate of disappearance of A
rate of formation of C is twice the rate of disappearance of A
Consider the following E° values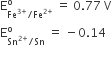
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) → 2Fe2+(aq) + Sn2+(aq) is
1.68 V
0.63 V
0.91 V
0.91 V
The molar solubility product is Ksp. ‘s’ is given in terms of Ksp by the relation
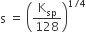

s = (256 Ksp)1/5
s = (256 Ksp)1/5
The standard e.m.f of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96,500 C mol-1: R = 8.314 JK-1 mol-1)
1.0×101
1.0×1030
1.0×1030
1.0×1030
The limiting molar conductivities Λ° for NaCl, KBr and KCl are 126, 152 and 150 S cm2 mol-1 respectively. The Λ° for NaBr is
128 S cm2 mol-1
302 S cm2 mol-1
278 S cm2 mol-1
278 S cm2 mol-1
In a cell that utilises the reaction Zn(s) + 2H+ (aq) → Zn2+(aq) + H2(g) addition of H2SO4 to cathode compartment, will
lower the E and shift equilibrium to the left
increases the E and shift equilibrium to the left
increase the E and shift equilibrium to the right
increase the E and shift equilibrium to the right
Which one the following statement regarding helium is incorrect?
It is used to fill gas balloons instead of hydrogen because it is lighter and non – inflammable
It is used in gas – cooled nuclear reactors
It is used to produce and sustain powerful superconducting reagents
It is used to produce and sustain powerful superconducting reagents
