 Multiple Choice Questions
Multiple Choice QuestionsThe enthalpies of combustion of carbon and carbon monoxide are -393.5 and -283 kJ mol-1 respectively. The enthalpy of formation of carbon monoxide per mole is
110.5 kJ
-110.5 kJ
-676.5 kJ
-676.5 kJ
B.
-110.5 kJ
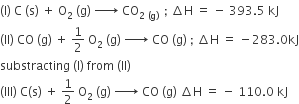
The ammonia evolved from the treatment of 0.30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0.1 M sulphuric acid. The excess of acid required 20 mL of 0.5 M sodium hydroxide solution hydroxide solutio for complete neutralization. The organic compound is
acetamide
thiourea
urea
urea
The smog is essentially caused by the presence of
O2 and O3
O3 and N2
Oxides of sulphur and nitrogen
Oxides of sulphur and nitrogen
Which one of the following aqueous solutions will exhibit highest boiling point?
0.01 M Na2SO4
0.015 M glucose
0.015 M urea
0.015 M urea
Which among the following factors is the most important in making fluorine the strongest oxidizing halogen?
Electron affinity
Bond dissociation energy
Hydration enthalpy
Hydration enthalpy
6.02×1020 molecules of urea are present in 100 ml of its solution. The concentration of urea solution is
0.001 M
0.1 M
0.02 M
0.02 M
To neutralize completely 20 mL of 0.1 M aqueous solution of phosphorous acid (H3PO3), the volume of 0.1 M aqueous KOH solution required is
10 mL
60 mL
40 mL
40 mL
For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values?
Heat of vaporization
Gaseous densities at the same temperature and pressure
Boiling points
Boiling points
Which of the following liquid pairs shows a positive deviation from Raoult’s law?
Water – hydrochloric acid
Acetone – chloroform
Water – nitric acid
Water – nitric acid
Which one of the following statements is false?
Raoult’s law states that the vapour pressure of a components over a solution is proportional to its mole fraction
Two sucrose solutions of same molality prepared in different solvents will have the same freezing point depression
The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 > KCl > CH3COOH > sucrose
The correct order of osmotic pressure for 0.01 M aqueous solution of each compound is BaCl2 > KCl > CH3COOH > sucrose
