 Multiple Choice Questions
Multiple Choice QuestionsHow many moles of magnesium phosphate, Mg3(PO4)2 will contain 0.25 mole of oxygen atoms?
0.02
3.125 × 10–2
1.25 × 10–2
1.25 × 10–2
According to Bohr’s theory, the angular momentum of an electron in 5th orbit is
25h/ π
1.0h/π
10/π
10/π
Uncertainty in the position of an electron (mass = 9.1 × 10–31 kg) moving with a velocity 300 ms–1, accurate upto 0.001%, will be
19.2 × 10–2 m
5.76 × 10–2 m
1.92 × 10–2 m
1.92 × 10–2 m
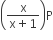
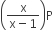
Phosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
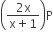

The standard enthalpy of formation (∆fHo) at 298 K for methane, CH4(g), is –74.8 kJ mol–1. The additional information required to determine the average energy for C – H bond formation would be
the dissociation energy of H2 and enthalpy of sublimation of carbon
latent heat of vapourization of methane
the first four ionization energies of carbon and electron gain enthalpy of hydrogen
the first four ionization energies of carbon and electron gain enthalpy of hydrogen
Among the following mixtures, dipole-dipole as the major interaction is present in
benzene and ethanol
acetonitrile and acetone
KCl and water
KCl and water
Which one of the following sets of ions represents a collection of isoelectronic species?
K+ , Cl– , Ca2+, Sc3+
Ba2+, Sr2+, K+ , S2–
N3–, O2–, F– , S2–
N3–, O2–, F– , S2–
In which of the following molecules/ions are all the bonds not equal?
SF4
SiF4
XeF4
XeF4
Nickel (Z = 28) combines with a uninegative monodentate ligand X–
to form a paramagnetic complex [NiX4 ]2 −. The number of unpaired electron(s) in the nickel and geometry of this complex ion are,
respectively
one, tetrahedral
two, tetrahedral
one, square planar
one, square planar