 Multiple Choice Questions
Multiple Choice QuestionsIn a hydrogen-oxygen fuel cell, combustion of hydrogen occurs to
generate heat
create potential difference between the two electrodes
produce high purity water
remove adsorbed oxygen from electrode surfaces
Amongst the following compounds, the optically active alkane having lowest molecular mass is
CH3-CH2-CH2-CH3
CH3-CH2-CH(CH3)-CH3
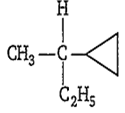
CH3-CH2-C≡CH
Which of the following will have a meso-isomer also?
2-chlorobutane
2,3-dichlorobutane
2,3-dichloropentane
2-hydroxypropanoic acid
The smog is essentially caused by the presence of
O2 and O3
O2 and N2
oxides of sulphur and nitrogen
O3 and N2
In this reaction
CH3CHO + HCN → CH3CH(OH)CN CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would be
50% D + 50% L-isomer
20% D + 80% L-isomer
D-isomer
L-isomer
Which one of the following octahedral complexes will not show geometrical isomerism? (A and B are monodentate ligands)
[MA4B2]
[MA5B]
[MA2B4]
[MA3B3]
B.
[MA5B]
[MA5B] due to absence of symmetry of 'B' ligand cannot exist in the form of cis-trans isomer.
According to IUPAC nomenclature sodium nitroprusside is named as
sodium pentacyanonitrosyl ferrate (II)
sodium pentacyanonitrosyl ferrate (III)
sodium nitroferricyanide
sodium nitroferrocyanide
Which of the following is responsible for depletion of the ozone layer in the upper strata of the atmosphere ?
Polyhalogens
Ferrocenes
Fullerenes
Freons
How many unit cells are present in a cube shaped ideal crystal of NaCl of mass 1.00 gm? [Atomic masses : Na = 23; Cl = 35.5]
2.57 × 1021
5.14 × 1021
1.28 × 1021
1.71 × 1021
