 Multiple Choice Questions
Multiple Choice QuestionsAn organic compound with molecular formula C6H12 upon ozonolysis give only acetone as the product. The compound is
2,3-dimethyl-1-butene
3-hexane
2-hexene
2,3-dimethyl-2-butene
Acyclic stereoisomer having the molecular formula C4H7Cl are classified and tabulated. Find out the correct set of numbers
| Geometrical | optical |
| 6 | 2 |
| Geometrical | optical |
| 4 | 2 |
| Geometrical | optical |
| 6 | 0 |
| Geometrical | optical |
| 6 | 0 |
A.
| Geometrical | optical |
| 6 | 2 |
The acyclic stereo isomers of C4H7Cl are
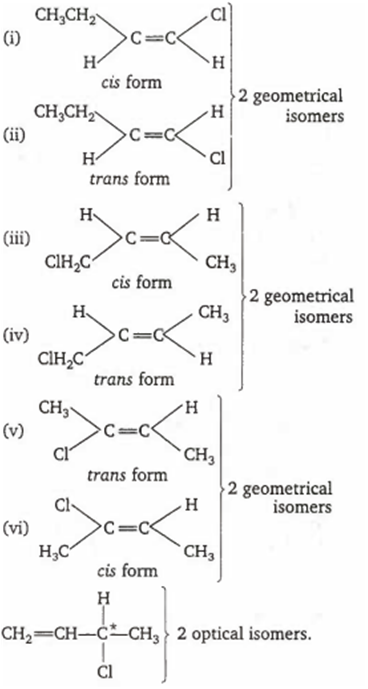
Number of optical isomers = 2n = 21 = 2
Hence, total number of geometrical isomers = 6
Total number of optical isomers = 2
Which of the following is used as an oxidiser in rocket propellants?
Ammonium perchlorate
Alcohol
Acrylic rubber
Polyurethane
A solid compound contains X, Y and Z atoms in a cubic lattice with X atom occupying the comers. Y atoms in the body centred positions and Z atoms at the centres of faces of the unit cell. What is the empirical formula of the compound?
XYZ
X2Y2Z3
XYZ3
XY2Z3
KCl crystallises in the same type of lattice as does NaCl. Given that rNa+/ rCl-= 0.55 and rK+/rCl-= =0.74. Calculate the ratio of the side of the unit cell for KCl to that of NaCl.
1.123
0.0891
1.414
0.414
Which one of the following reactions will occur on heating AgNO3 above its melting point?
2AgNO3 → 2Ag + 2NO2 + O2
2AgNO3 → 2Ag + N2 + 3O2
2AgNO3 → 2AgNO2 + O2
2AgNO3 → 2Ag + 2NO + 2O2
Pick out the correct statements from the following
1.Cobalt (III) is more stable in octahedral complexes.
2. Zinc forms coloured ions or complexes.
3. Most of the d-block elements and their compounds are ferromagnetic.
4. Osmium shows (VIII) oxidation state.
5. Cobalt (II) is more stable in octahedral complex.
1 and 2
1 and 3
2 and 4
1 and 4
