 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compound(s) has 'Z' configuration ?
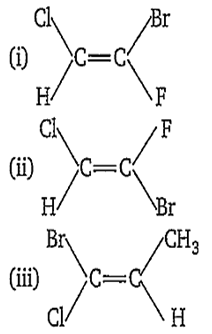
(i) only
(ii) only
(iii) only
(i) and (iii) only
According to Cahn-Ingold-Prelog sequence rules, the correct order of priority for the given groups is
-COOH > -CH2OH > -OH > -CHO
-COOH > -CHO > -CH2OH > -OH
-OH > -CH2OH > -CHO > -COOH
-OH > -COOH > -CHO > -CH2OH
What are X and Y respectively in the following reaction?
Z-product 2-butyne E-product
Na/ NH3 (liq) and Pd/ BaSO4 + H2
Ni/ 140°C and Pd/ BaSO4 + H2
Ni/ 140°C and Na/ NH3 (liq)
Pd/ BaSO4 + H2 and Na/ NH3 (liq)
In which of the following reactions, chlorine acts as an oxidising agent?
(i) CH3CH2OH + Cl2 → CH3CHO + HCl
(ii) CH3CHO + Cl2 → CCl3 . CHO + HCl
(iii) CH4 + Cl2 CH3Cl + HCl
The correct answer is-
(i) only
(ii) only
(i) and (iii)
(i), (ii) and (iii)
The correct order of reactivity of hydrogen halides with ethyl alcohol is
HF > HCl > HBr > HI
HCl > HBr > HF > HI
HBr > HCl > HI > HF
HI > HBr > HCl > HF
The IUPAC name of the following compound is-
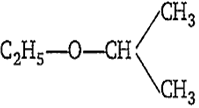
ethoxy propane
1,1-dimethyl ether
2-ethoxy isopropane
2-ethoxy propane
The structure of the compound formed, when nitrobenzene is reduced by lithium aluminium hydride (LiAlH4) is
![]()
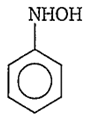
![]()
![]()
If the mass defect of 5B11 is 0.081 u, its average binding energy (in MeV) is
8.60
6.85
5.60
5.86
What is the temperature at which the kinetic energy of 0.3 moles of helium is equal to the kinetic energy of 0.4 moles of argon at 400 K?
400 K
873 K
533 K
300 K
Assertion (A) : The aqueous solution of CH3COONa is alkaline in nature.
Reason (R) : Acetate ion undergoes anionic hydrolysis.
The correct answer is
both (A) and (R) are true and (R) is the correct explanation of (A).
both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true but (R) is not true.
A) is not true but (R) is true.
A.
both (A) and (R) are true and (R) is the correct explanation of (A).
CH3COONa + H2O → CH3COOH + NaOH
The above process takes place in following steps-
CH3COONa + OH-
CH3COO- + H2O → CH3COOH + OH-
Acetate ion undergoes anionic hydrolysis and the resulting solution is slightly basic due to excess of OH- ions. Hence, both (A) and (R) are true and (R) is the correct explanation of (A).
