 Multiple Choice Questions
Multiple Choice QuestionsCorrect order of electron affinities of halogens is
F> Cl> Br> I
I> Br> Cl> F
Cl> F>I>Br
Cl>F>Br>I
If NO2(N2O4) is dissolved in NaOH, we get solution of
NaNO2
NaNO3
mixture of NaNO2 and NaNO3
NaNO4
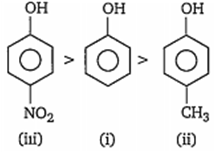
Correct acidic order of the following compounds is

i > ii> iii
iii> i> ii
ii> iii> i
i> iii> ii
B.
iii> i> ii
Presence of electron withdrawing group such as NO2 CHO etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxde ion while presence of electron releasing groups such as -CH3, -C2H5, destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compounds is
Which of the following is strongest base?
Perkin's reaction
Knoevenagel reaction
Reformatsky reaction
Benzoin condensation
Which of the following will not respond to iodoform test?
Ethyl alcohol
Propanol-2
Propanol-1
Ethanal
Which of the following acids will have lowest value of pKa?
CH3CH2COOH
CH3CHBrCOOH
CH3CHFCOOH
FCH2CH2COOH
Which of the following does not contain chiral carbon atom?
Lactic acid
2-chlorobutanoic acid
Tartaric acid
Succinic acid
What will be the compound if two valencies of carbonyl group are satisfied by two alkyl groups?
Aldehyde
Ketone
Acid
Acidic anhydride
