 Multiple Choice Questions
Multiple Choice QuestionsOctane number of petrol can be improved by admixing it with which of the following chemicals?
Et4Pb
CH3OC(CH3)3
Both (a) and (b)
Pyrene
Cyclohexane, methylcyclopentane, 1, 3-dimethyl cyclobutane and 1, 2, 3-trimethyl cyclopropane are examples of which one of the following?
Constitutional isomers
Structural isomers
Both (a) and (b)
Structural as well as positional isomers
How many structural isomers are possible for a molecule of C6H12,composition but having at least-one carbocyclic ring?
Seven
Six
Five
None of these
The configurations of the chiral centres in A and B are, respectively, as
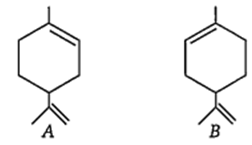
R and S
S and R
R and R
S and S
B.
S and R

Here * indicates chiral carbon atom.
Arrange Na+, Mg+ and Al3+ in increasing order of energy of hydration.
Na+ < Mg+ < Al3+
Al3+< Mg+ < Na+
Al3+ < Na+ < Mg2+
Mg2+< Al3+ < Na+
You may have noticed that chicken-egg is very strong along its long axis so much so that it does not break even when pressed very hard. Which of the following is the main constituent of egg-shell?
CaSO4·2H2O
CaSO4·1/2H2O
CaSiO3
CaCO3
Nuclear attraction is often the deciding control factor for the association of neutral molecules to a given metal ion. Which one of the following represents the correct order of stability of the ions?
[Be(H2O)4]2+, [Mg(H2O)4]2+, [Ca(H2O)4]2+ and [Sr(H2O)4]2+ ?
[Be(H2O)4]2+ > [Sr(H2O)4]2+> [Mg(H2O)4]2+ > [Ca(H2O)4]2+
[Ca(H2O)4]2+> [Mg(H2O)4]2+ > Be(H2O)4]2+ > [Sr(H2O)4]2+
[Sr(H2O)4]2+ > [Ca(H2O)4]2+> [Mg(H2O)4]2+ > Be(H2O)4]2+
[Be(H2O)4]2+> [Mg(H2O)4]2+ > [Ca(H2O)4]2+ > [Sr(H2O)4]2+
Which of the following represents correct acidity order Li2O , BeO and B2O3 ?
Li2O < BeO < B2O3
B2O3 < BeO < Li2O
BeO < Li2O < B2O3
BeO < B2O3 < Li2O
Which of the following does not/do not represent the correct equivalent mass of Cr2O in the reaction Cr2O+ 14 H+ + 6e- → 2Cr3+ + 7H2O.
The molar mass of dichromate
One third of the molar mass of dichromate
One half of the molar mass of dichromate
all of the above
