 Multiple Choice Questions
Multiple Choice QuestionsA piston filled with 0.04 mol of an ideal gas expands reversibly from 50.0 mL to 375 mL at a constant temperature of 37.00C. As it does so, it absorbs 208J of heat. The values of q and w for the process will be:(R = 8.314 J/mol K) ( ln 7.5 = 2.01)
q =+208J, W = - 208 J
q =-208 J, W =-208 J
q=-208J, W = +208 J
q=-208J, W = +208 J
For the gaseous state, if most probable speed is denoted by C*, average speed by C and mean square speed by C, then for a large number of molecules the ratios of these speeds are:
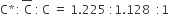
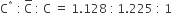
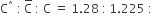
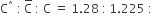
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g of CO2.The empirical formula of the hydrocarbon is
C2H4
C3H4
C6H5
C6H5
In which of the following pairs of molecules/ions, both the species are not likely exist?


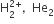
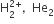
Which of the following exists as covalent crystals in the solid state?
Iodine
Silicon
Sulphur
Sulphur
Which of the following represents the correct order of increasing first ionisation enthalpy for Ca, Ba, S, Se and Ar?
Ca <S<Ba<Se<Ar
S< Se<Ca<Ba< Ar
Ba< Ca<Se< S< Ar
Ba< Ca<Se< S< Ar
Energy of an electron is given by
E =- 2.178 x 10-18 J 
Wavelength of light required to excite an electron in a hydrogen atom from level n =1 to n=2 will be (h=6.62 x 1034 Js and c = 3.0 x 108 ms-1)
1.214 x 10-7 m
2.816 x 10-7 m
6.500 x 10-7 m
6.500 x 10-7 m
The first ionisation potential of Na is 5.1 eV. The value of electron gain enthalpy of Na+ will be
-2.55 eV
-5.1 eV
-10.2 eV
-10.2 eV
Stability of the species Li2, Li2− and Li2+ increases in the order of:
Li2 < Li2+ < Li2-
Li2− < Li2+ < Li2
Li2< Li2− < Li2+
Li2< Li2− < Li2+
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in its oxide. The fraction of the metal which exists as M3+ would be:
7.01%
4.08%
6.05%
6.05%