 Multiple Choice Questions
Multiple Choice QuestionsConsider the following reaction,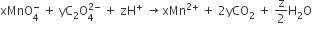
The values of x,y and z in the reaction are respectively
5,2 and 16
2,5 and 8
2,5 and 16
2,5 and 16
C.
2,5 and 16
The gas leaked from a storage tank of the Union Carbide plant in Bhopal tragedy was
Methyl isocyanate
Methylamine
Ammonia
Ammonia
Which of the following complex species is not expected to exhibit optical isomerism?
[Co(en)3]3+
[Co(en)2Cl2]+
[Co(NH3)3Cl3]
[Co(NH3)3Cl3]
Which one of the following molecules is expected to exhibit diamagnetic behaviour?
C2
O2
N2
N2
Given, 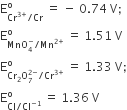
Based on the data given above, strongest oxidising agent will be
Cl
Cr3+
Mn2+
Mn2+
The molarity of a solution obtained by mixing 750 mL of 0.5 (M) HCl with 250 mL of 2(M) HCl will be
0.875 M
1.00 M
1.75 M
1.75 M
The rate of a reaction doubles when its temperature changes from 300K to 310K. Activation energy of such a reaction will be (R = 8.314 JK–1 mol–1 and log 2 = 0.301)
53.6 kJ mol-1
48.6 kJ mol-1
58.5 kJ mol-1
58.5 kJ mol-1
Which of the following arrangements does not represent the correct order of the property stated against it?
V2+ < Cr2+<Mn2+<Fe2+: paramagnetic behaviour
Ni2+ < Co2+ < Fe2+ < Mn2+ ; Ionic size
Co3+ < Fe3+< Cr3+ < Sc3+ : stability in aqueous solution
Co3+ < Fe3+< Cr3+ < Sc3+ : stability in aqueous solution
Which of the following is the wrong statement?
ONCl and ONO- are not isoelectronic
O3 molecule is linear
Ozone is violet-black in solid state
Ozone is violet-black in solid state
The coagulating power of electrolytes having ions Na+, Al3+ and Ba2+ for arsenic sulphide sol increases in the order:
Al3+<Ba2+<Na+
Na+<Ba2+<Al3+
Ba2+< Na2+<Al3+
Ba2+< Na2+<Al3+
