 Multiple Choice Questions
Multiple Choice Questions
The product Y is
p-chloro nitrobenzene
o-chloro nitrobenzene
m-chloro nitrobenzene
o, p-dichloro nitrobenzene
Following compounds are respectively ..... geometrical isomers
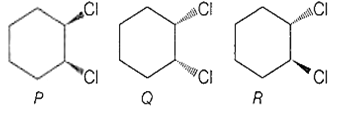
P - cis ;Q - cis ; R - trans
P - cis ; Q - trans ; R - trans
P - trans ; Q - cis ; R - cis
P - cis ; Q - trans ; R - cis
Which is more basic oxygen in an ester?
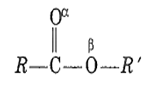
Carbonyl oxygen, α
Carboxyl oxygen, β
Equally basic
Both are acidic oxygen
Match the following and choose the correct option given below:-
| Compound / Type | Use |
| A. Dry ice | I. Anti-knocking compound |
| B. Semiconductor | II. Electronic diode or triode |
| C. Solder | III. Joining circuits |
| D. TEL | IV. Refrigerant for preserving food |
A - I; B - II; C - IV; D - III
A - II; B - III; C - I ; D - IV
A - IV; B - III; C - II; D - I
A - IV; B - II; C - III; D - I
B can be obtained from halide by van- Arkel method. This involves. This involves reaction
2BI3 2B + 3I2
2BCl3 + 3H2 2B +6HCl
Both (a) and (b)
None of the above
A.
2BI3 2B + 3I2
In van-Arkel method, pyrolysis of BI3 is carried out in the presence of red hot W or Ta filament.
2BI3 2B + 3I2
The density of solid argon is 1.65 g per cc at -233°C. If the argon atom is assumed to be a sphere of radius 1.54 × 10-8 cm, what percent of solid argon is apparently empty space? (Ar = 40)
16.5%
38%
50%
62%
Acid hydrolysis of ester is first order reaction and rate constant is given by
k =
where, V0, Vt and V∞ are the volume of standard NaOH required to neutralise acid present at a given time, if ester is 50% neutralised then
V∞ = Vt
V∞ = (Vt - V0)
V∞ = 2Vt - V0
V∞ = 2Vt + V0
Which of these ions is expected to be coloured in aqueous solution?
I. Fe3+
II. Ni2+
III. Al3+
I and II
II and III
I and III
I, II and III
Consider the following reaction,

The reaction is of first order in each diagram, with an equilibrium constant of 104. For the conversion of chair form to boat form = 4.5 × 10-8 m at 298 K with pre-exponential factor of 1012 s-1. Apparent rate constant (=kA/ kB) at 298 K is
4.35 × 104 s-1
4.35 × 108 s-1
4.35 × 10-8 s-1
4.35 × 1012s-1
