 Multiple Choice Questions
Multiple Choice QuestionsThe molecular formula of a commercial resin used for exchanging ions in water softening is C8H7SO3Na ( mol. wt = 206). What would be the maximum uptake of Ca2+ ions by the resin when expressed in mole per gram resin?
1/103
1/206
2/309
2/309
Which of the following is the energy of a possible excited state of hydrogen?
+13.6 eV
-6.8 eV
-3.4 eV
-3.4 eV
The intermolecular interaction that is dependent on the inverse cube of the distance between the molecule is:
ion-ion interaction
ion-dipole interaction
London force
London force
The following reaction is performed at 298 K
2NO(g) + O2 (g) ⇌ 2NO2 (g)
The standard free energy of formation of NO (g) is 86.6 kJ/mol at 298 K. What is the standard free energy of formation of NO2 (g) at 298 K? (KP = 1.6 x 1012)
R (298) In (1.6 x 1012)-86600
86600 + R (298) In (1.6 x 1012)
86600 - In(1.6 x 1012)/R(298)
86600 - In(1.6 x 1012)/R(298)
The standard Gibbs energy change at 300 K for the reaction, 2A ⇌ B +C is 2494.2J at a given time, the composition of the reaction mixture is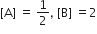 and
and 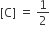 , The reaction proceeds in the [R= 8.314 JK/mol, e = 2.718]
, The reaction proceeds in the [R= 8.314 JK/mol, e = 2.718]
forward direction because Q>Kc
reverse direction because Q>Kc
forward direction because Q < Kc
forward direction because Q < Kc
The ionic radii (in Å) of N3–, O2– and F– are respectively:
1.36, 1.40 and 1.71
1.36, 1.71 and 1.40
1.71, 1.40 and 1.36
1.71, 1.40 and 1.36
In Carius method of estimation of halogens, 250 mg of an organic compound gave 141 mg of AgBr. The percentage of bromine in the compound is: (at. Mass Ag = 108; Br = 80)
24
36
48
48
From the following statements regarding H2O2, choose the incorrect statement:
It can act only as an oxidising agent
It decomposed on exposure to light
It has to be stored in plastic or wax lined glass bottles in dark
It has to be stored in plastic or wax lined glass bottles in dark
Which one of the following alkaline earth metal sulphates has its hydration enthalpy greater than its lattice enthalpy?
CaSO4
BeSO4
BaSO4
BaSO4
Sodium metal crystallises in a body centred cubic lattice with a unit cell edge of 4.29 Å The radius of sodium atom is approximate:
1.86 Å
3.22Å
5.72 Å
5.72 Å