Multiple Choice Questions
Multiple Choice QuestionsThe correct order of increasing C-O bond length of CO, CO2 and CO is
CO < CO2 < CO
CO2 < CO < CO
CO < CO < CO2
CO < CO2 < CO
A sample of gasoline contain 81% octane and 19% n-heptane. Its octane, number will be
19
81
100
62
The IUPAC name of the following compound is:
![]()
3-methyl cyclohexene
1-methyl cyclohex-2-ene
6-methyl cyclohexene
1-methyl cyclohex-5-ene
Which of the following does not exist?
I. HO3S-S-SO3H
II. HO-Te-OH
III. HS-S2-SH
IV. HS-Po-OH
Only II
Only III
II and IV
I, III and IV
Acidic dichromate ion reacts with hydrogen peroxide to give deep blue colour. This is due to the formation of
CrO(O)2
CrO5
Bith (a) and (b)
None of (a) and (b)
The coordination number and oxidation number X in the following compound [X(SO4)(NH3)5]Cl will be
10 and 3
2 and 6
6 and 3
6 and 4
Liquid hydrocarbon is converted into mixture of gaseous hydrocarbons by
cracking
oxidation
hydrolysis
distillation under reduced pressure
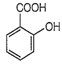
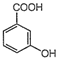
Among the following compound with the lowest pKa value is
![]()
![]()
A.

Strength of acid is indicated by pKa value. Higher the value of Ka or lower the value of pKa, stronger is the acid. Among the given aromatic acids, the strength decreases as follows: