 Multiple Choice Questions
Multiple Choice QuestionsSelect the correct statement for thermoplastic polymer.
It does not become soft on heating under pressure
It cannot be remoulded
It is either linear or branched chain polymer
It is a cross- linked polymer.
The amine 'A' when treated with nitrous acid gives yellow oily substance. The amine A is
triethylamine
trimethylamine
aniline
methylphenylamine
lsopropyl methyl ether when treated with cold hydrogen iodide gives
isopropyl iodide and methyl iodide
isopropyl alcohol and methyl iodide
isopropyl alcohol and methyl alcohol
isopropyl iodide and methyl alcohol
Glucose on oxidation with bromine water yields gluconic acid. This reaction confirms the presence of
six carbon atoms linked in straight chain
secondary alcoholic group in glucose
aldehyde group in glucose
primary alcoholic group in glucose
C.
aldehyde group in glucose
Glucose on oxidation with bromine water yields gluconic acid. This reaction confirms the presence of aldehyde group in glucose.
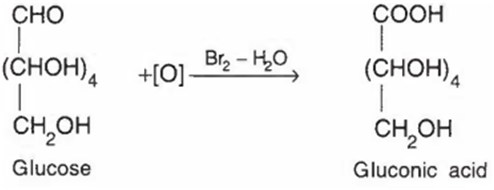
Here, Br2-H2O act as an oxidising agent and led to the oxidation of glucose into gluconic acid.
Select the compound which on treatment with nitrous acid liberates nitrogen.
Nitroethane
Triethylamine
Diethylamine
Ethylamine
