 Multiple Choice Questions
Multiple Choice QuestionsThe ion which is not tetrahedral in shape is
BF4-
NH4+
Cu(NH3)42+
NiCl42-
C.
Cu(NH3)42+
Among all the given options, Cu(NH3)42+ is not tetrahedral in shape. Its electronic configuration is 3d9 4s0.
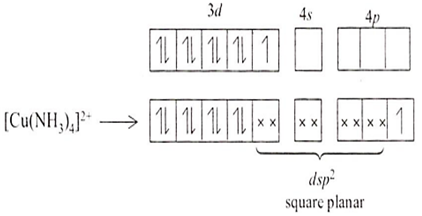
One electron is shifted from 3d to 4p orbital.
MnO (1 mole) in neutral aqueous medium is disproportionated to
2/3 mole of MnO and 1/3 mole of MnO2
1/3 mole of MnO and 2/3 mole of MnO2
1/3 mole of Mn2O7 and 1/3 mole of MnO2
2/3 mole of Mn2O7 and 1/3 mole of MnO2
Which of the following are arranged in the decreasing order of dipole moment?
CH3Cl, CH3Br, CH3F
CH3Br, CH3Cl, CH3F
CH3Cl, CH3Br, CH3F
CH3Br, CH3F, CH3Cl
Which of the following compounds possesses the C-H bond with the lowest bond dissociation energy?
Toluene
Benzene
n-Pentane
2,2-Dimethylpropane
One gram sample of NH4NO3 is decomposed in a bomb calorimeter. The temperature of the calorimeter increases by 6.12 K. The heat capacity of the system is 1.23 kJ/g/deg. What is the molar heat of decomposition for NH4NO3 ?
-7.53 kJ/mol
-398.1 kJ/mol
-16.1 kJ/mol
-602 kJ/mol
Which one of the following has S° greater than zero?
CaO(s) + CO2(g) CaCO3(s)
NaCl(aq) NaCl(s)
NaNO3(s) Na+(aq) + NO3-(aq)
N2(g) + 3H2(g) 2NH3(g)
