 Multiple Choice Questions
Multiple Choice QuestionsThe quantum number m of a free gaseous atom is associated with
the effective volume of the orbital
the shape of the orbital
the spatial orientation of the orbital
the energy of the orbital in the absence of a magnetic field.
C.
the spatial orientation of the orbital
The quantum number m of a free gaseous atom is associated with the spatial orientation of the orbital. It describes the orientation or distribution of electron cloud.
In which of the following acid-base titration, pH is greater than 8 at equivalence point?
Acetic acid versus ammonia
Acetic acid versus sodium hydroxide
Hydrochloric acid versus ammonia
Hydrochloric acid versus sodium hydroxide
The potential energy diagram for a reaction R → P is given in the figure. H ° of the reaction corresponds to the energy
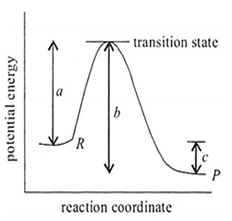
a
b
c
a + b
Assertion : NF3 is a weaker ligand than N(CH3)3 .
Reason : NF3 ionizes to give F- ions in aqueous solution.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : emits a positron giving .
Reason : In β+ emission neutron is transformed into proton.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : Barium is not required for normal biological function in human.
Reason : Barium does not show variable oxidation state.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : The increase in internal energy (E) for the vapourization of one mole of water at 1 atm and 373 K is zero.
Reason : For all isothermal processes, E = 0.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : BaCO3 is more soluble in HNO3 than in plain water.
Reason : Carbonate is a weak base and reacts with the H+ from the strong acid, causing the barium salt to dissociate.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : H and E are almost the same for the reaction, N2(g) + O2(g) → 2NO(g)
Reason : All reactants and products are gases.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Assertion : The O - O bond length in H2O2 is shorter than that of O2F2.
Reason : H2O2 is an ionic compound.
If both assertion and reason are true and the reason is the correct explanation of the assertion.
If both assertion and reason are true and the reason is not the correct explanation of the assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
