 Multiple Choice Questions
Multiple Choice QuestionsDimethyl glyoxime gives a red precipitate with Ni2+, which is used for its detection. To get this precipitate readily the best pH range is
< 1
2-3
3- 4
9- 11
The statement true for is
it has a non-linear structure
it is called pseudohalogen
the formal oxidation state of nitrogen in this anion is -1
it is isoelectronic with NO2
The compound insoluble in water is
mercurous nitrate
mercuric nitrate
mercurous chloride
mercurous perchlorate.
The ONO angle is maximum in
D.
The nitrate ion has three resonance structure, with the double bond on a different oxygen for each. In all resonance structures the nitrogen has three bonds, therefore, its hybridisation is sp2. Each of the oxygen atoms also has sp2 hybridisation, since the hybridisation is determined by the resonance structure with the double bond, where the oxygen has a double bond and two lone pairs.
The actual geometry of the polyatomic ion is trigonal planar with bond angles of 120°,
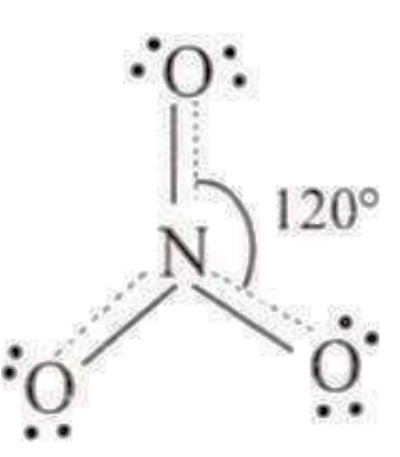
:18 electrons; ideal geometry trigonal planar, sp2 with bond angle of 116°.
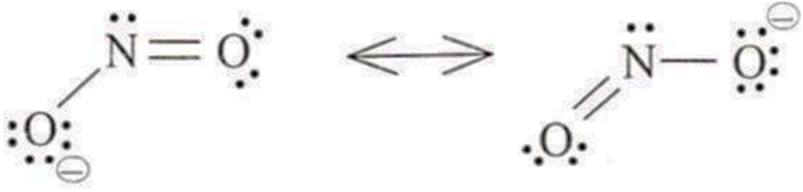
NO2 : 17 electrons, ideal geometry trigonal planar; sp2 with bond angle of 134°.
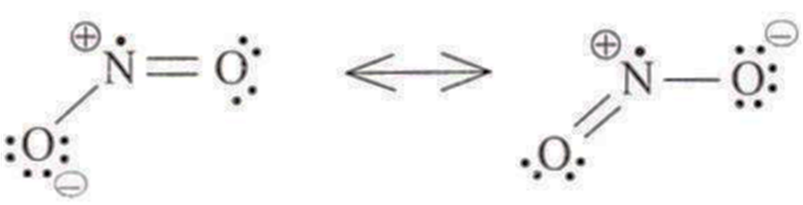
: 16 electrons; ideal geometry linear ; sp with bond angle of 180°,
How much energy is released when 6 moles of octane is burnt in air? Given for CO2(g) , H2O(g) and C8H18(l) respectively are - 490, -240 and +160 kJ/mol.
-6.2 kJ
-37.4 kJ
-35.5 kJ
-20.0 kJ
For the equilibrium at 1 atm and 298K.
standard free energy change is equal to zero (= 0)
free energy change is less than zero ( < 0)
standard free energy change is less than zero (< 0)
standard free energy change is greater than zero (G° > 0).
