 Multiple Choice Questions
Multiple Choice QuestionsAmong the following molecules
(i) eO3 (ii) eOF4 (iii) eF6
those having same number of lone pairs on Xe are
(i) and (ii) only
(i) and (iii) only
(ii) and (iii) only
(i), (ii) and (iii)
emits 8 α-particles and 6 β-particles. The neutron/proton ratio in the product nucleus is
60/41
61/40
62/41
61/42
α-Particles can be detected using
thin aluminium sheet
barium sulphate
zinc sulphide screen
gold foil
(298 K) of methanol is given by the chemical equation
CH4 (g) + O2 (g) →CH3OH(g)
C (graphite) + O2 (g) + 2H2 → CH3OH(l)
C (diamond) + O2(g) + 2H2(g) → CH3OH(l)
CO(g) + 2H2 → CH3OH(l)
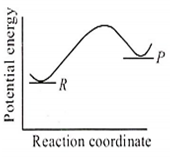
An endothermic reaction with high activation energy for the forward reaction is given by the diagram



When 10 mL of 0.1 M acetic acid (pKa = 5.0) is titrated against 10 ml of 0.1 M ammonia solution (pKb = 5.0), the equivalence point occurs at pH
5.0
6.0
7.0
9.0