 Multiple Choice Questions
Multiple Choice QuestionsIf the Zn2+/Zn electrode is diluted to 100 times then the change in emf :
increase of 59 mV
decrease of 59 mV
increase of 29.5 mV
decrease of 29.5 mV
If equivalent conductance of 1 M benzoic acid is 12.8 ohm-1 cm and if the conductance of benzoate ion and H+ ion are 42 and 288 .42 ohm-1 cm2 respectively .Its degree of dissociation is :
39 %
3.9 %
0.35 %
0.039 %
If two substances A and B have : = 1 : 2 and have mole fraction in solution 1 : 2 then mole fraction of A in vapours :
0.33
0.25
0.52
0.2
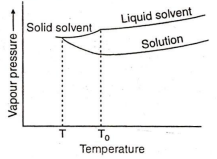
If an aqueous solution of glucose is allowed to freeze then crystal of which will be separated out first ?
Glucose
Water
Both (a) and (b)
None of these
B.
Water
The freezing point of a. liquid is that temperature at which the liquid and its solid state exist in equilibrium with each other and have same vapour pressure .Due to lower vapour pressure of the solution , solid form of solution separates out a lower temperature .The decrease in freezing point is called depression in freezing point .Thus if an aqueous solution of glucose is allowed to freeze then crystal of water will be separated out first .
A + 2B C + D , if
- = 5 x 10-4 mol L-1 s-1 , then - is
2.5 x 10-4 mol L-1 s-1
50 x 10-4 mol L-1 s-1
2.5 x 10-3 mol L -1 s-1
1.0 x 10-3 mol L -1 s-1
Which statement is wrong for NO ?
It is anhydride of nitrous acid
Its dipole moment is 0.22 D
It forms dimer
It is paramagnetic
