 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of acid strength is
HClO < HClO2 < HClO3 < HClO4
HClO4 < HClO < HClO2 < HClO3
HClO2 < HClO3 < HClO4 < HClO
HClO4 < HClO3 < HClO2 < HClO
The main reason for larger number of oxidation states exhibited by the actinides than the corresponding lanthanides, is
lesser energy difference between 5f and 6d orbitals than between 4f and 5d orbitals
larger atomic size of actinides than the lanthanide
more energy difference between 5f and 6d orbitals than between 4f and 5d orbitals
greater reactive nature of the actinides than the lanthanides
Aniline in a set of reactions yielded a product D
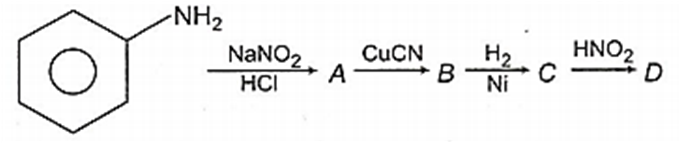
The structure of the product D would be
C6H5CH2NH2
C6H5NHCH2CH3
C6H5NHOH
C6H5CH2OH
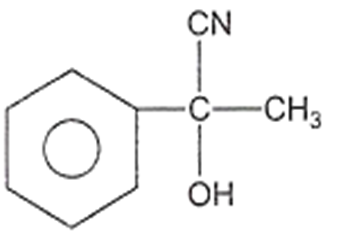
In a set of reactions, acetic acid yielded a product D.
CH3COOH A B C D
The structure of D would be
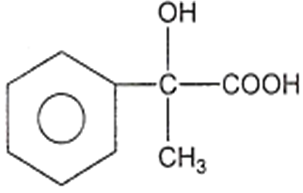
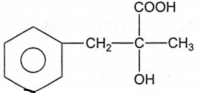
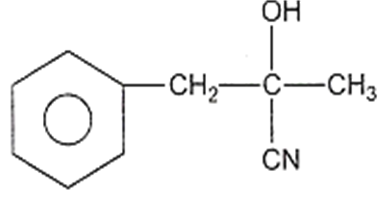
Electrolytic reduction of nitrobenzene in weakly acidic medium gives
aniline
nitrosobenzene
N-phenylhydroxylamine
p-hydroxyaniline
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
Benzyl chloride
Ethyl chloride
Chlorobenzene
lsopropyl chloride
Which functional group participates in disulphide bond formation in proteins?
Thiolactone
Thiol
Thioether
Thioester
B.
Thiol
Disulphide bond may be reduced to thiol by means of reagents, such as, NaBH4, which shows the presence of thiol group in disulphide bond formation.
