 Multiple Choice Questions
Multiple Choice QuestionsThe pH of the solution obtained on neutralisation of 40 mL 0.1 M NaOH with 40 mL 0.1 M CH3COOH is
7
8
6
3
Inert gases are mixed in iodine vapours. Then there are _____ between them.
H-bonding
van der Waals forces
electrostatic forces
metallic bonds
In P versus V graph, the horizontal line is found in which exists
Gas
Liquid
Equilibrium between gas and liquid
Super critical temperature
During estimation of nickel, we prepare nickel dimethylglyoxime, a scarlet red solid. This compound is
ionic
covalent
metallic
non-ionic complex
Critical temperatures for A, B, C and D gases are 25°C, 10°C, -80°C and 15°C respectively. Which gas will be liquefied more easily?
A
B
C
D
During titration of acetic acid with aqueous NaOH solution, the neutralisation graph has a vertical line. This line indicates
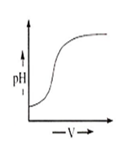
alkaline nature of equivalence
acidic nature of equivalence
neutral nature of equivalence
depends on experimental proceeding
Calculate change in internal energy if H = - 92.2 kJ, P = 40 atm and V = -1L.
-42 kJ
-88 kJ
+88 kJ
+42 kJ