 Multiple Choice Questions
Multiple Choice QuestionsEqual volumes of three acid solutions of pH 3.4 and 5 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
1.11 x 10-4 M
3.7 x 10-4 M
3.7 x 10-3 M
3.7 x 10-3 M
The angular shape of ozone molecule (O3) consists of
1 sigma and 2 pi bonds
2 sigma and 2 pi bonds
1 sigma and 1 pi bonds
1 sigma and 1 pi bonds
Which of the following are not state functions?
I) q + W
II) q
III) W
IV) H-TS
(I) and (IV)
(II) (III) and (IV)
(I), (II) and (III)
(I), (II) and (III)
The correct order of decreasing second ionisation enthalpy of Ti (22), V (23), Cr(24) and Mn (25) is
Cr > Mn> V > Ti
V > Mn > Cr >Ti
Mn > Cr> Ti >V
Mn > Cr> Ti >V
If a gas expands at constant temperature, it indicates that
the kinetic energy of molecules decrease
the pressure of the gas increases
the kinetic energy of molecules remains the same
the kinetic energy of molecules remains the same
For the gas phase, reaction,
PCl5 (g) ⇌ PCl3 (g) Cl2 (g)
Which of the following conditions are correct?
Δ H = 0 and ΔS > 0
ΔH >0 and ΔS > 0
Δ H < 0 and ΔS < 0
Δ H < 0 and ΔS < 0
Number of moles of MnO4- required to oxidies one mole of ferrous oxalate completely in acidic medium will be
0.6 mole
0.4 mole
7.5 mole
7.5 mole
A.
0.6 mole
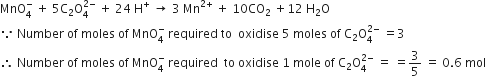
If the concentration of OH- ions in the reaction Fe(OH)3 (s) ⇌ Fe3+ (aq) + 3OH-(aq) is decreased by 1/4 times, then equilibrium concentration of Fe3+ will increase by
8 times
16 times
64 times
64 times
The sequence of ionic mobility in aqueous solution is
K+ > Na+ > Rb+ > Cs+
Cs+ > Rb+ > K+ >Na+
Rb+ > K+> Cs+ > Na+
Rb+ > K+> Cs+ > Na+
