 Multiple Choice Questions
Multiple Choice QuestionsAssertion : Compressibility factor for hydrogen varies with pressure with positive slope at all pressure.
Reason : Even at low pressure, repulsive forces dominate for hydrogen gas.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
For a dilute solution, Raoult's law states that
the relative lowering of vapour pressure is proportional to the amount of solute in solution
the relative lowering of vapour pressure is equal to the mole fraction of solute
the lowering of vapour pressure is equal to the mole fraction of the solute
the vapour pressure of the solution is equal to the mole fraction of the solvent.
To a 25 mL H2O2 solution, excess of acidified solution of Kl was added. The iodine liberated required 20 ml of 0.3 N Na2S2O3 solution. The volume strength of H2O2 solution is
1.344 g/L
3.244 g/L
5.4 g/L
4.08 g/L
In a homogenous reaction, A B + C + D the initial pressure was P0 and after time t it was P. Expression for rate constant k in terms of P0, P and t will be
Which curve corresponds to the temperature dependence of the rate R of a simple one step reaction?
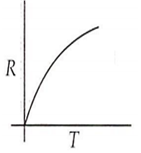
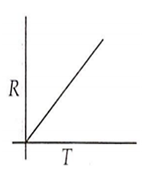
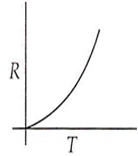
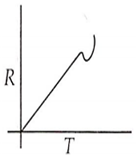
A 0.1 molal solution of an acid is 4.5% ionized. Calculate freezing point. (molecular weight of the acid is 300). Kf = 1.86 K mol-1 kg.
-0.194C
2.00C
0C
-0.269C
The specific conductance of a N/ 10 KCl at 25°C is 0.0112 ohm-1cm-1.The resistance of cell containing solution at the same temperature was found to be 55 ohm. The cell constant will be
6.16 cm-1
0.616 cm-1
0.0616 cm-1
616 cm-1
B.
0.616 cm-1
In the above given question,
Specific conductance = cell constant conductance
Therefore,
Cell constant = Resistance specific conductance
= 55 0.0112 = 0.616 cm-1
A mixture of two miscible liquids A and B is distilled under equilibrium conditions at 1 atm pressure. The mole fraction of A in solution and vapour phase are 0.30 and 0.60 respectively. Assuming ideal behaviour of the solution and the vapour, calculate the ratio of the vapour pressure of pure A to that of pure B.
4.0
3.5
2.5
1.85
Schottky defect in crystals is observed when
unequal number cations and anions are missing from the lattice
equal number of cations and anions are missing from the lattice
an ion leaves its normal site and occupies an interstitial site
density of the crystal is increased.
Assertion : In rate law, unlike in the expression for equilibrium constants, the exponents for concentrations do not necessarily match the stoichiometric coefficients.
Reason : It is the mechanism and not the balanced chemical equation for the overall change that governs the reaction rate.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false
