 Multiple Choice Questions
Multiple Choice QuestionsThe total number of atomic orbitals in fourth energy level of an atom is
16
32
4
8
A.
16
Number of atomic orbitals in an orbit is given by,
= n2 = 42 = 16
The number of atomic orbitals in fourth energy level is = 16
By what factor does the average velocity of a gaseous molecule increase when the temperature (in kelvin) is doubled?
2.8
4.0
1.4
1.4
In Duma's method of estimation of nitrogen 0.35 g of an organic compound gave 55 mL of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be
(Aqueous tension at 300 k = 15 mm)
16.45
17.45
14.45
14.45
Mole fraction of the solute in a 1.00 molal aqueous solution is
0.0177
0.0344
1.7700
1.7700
The value of ΔH for the reaction
X2 (g) + 4Y2 (g) ⇌ 2XY4 (g) is less than zero. Formation of XY4 (g) will be favoured at
Low pressure and low temperature
high temperature and low pressure
high pressure and low temperature
high pressure and low temperature
For the reaction N2 (g) + O2 (g) ⇌ 2NO (g), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction 2NO (g) + O2(g) ⇌ 2NO2 (g). What is K for the reaction 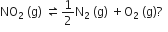
1/(4K1K2)
[1/K1K2]1/2
1/(K1K2)
1/(K1K2)
The energies E1 and E2 of two radiations are 25 eV respectively. The relation between their wavelength i.e., λ1 and λ2 will be
λ1 = 2λ2
λ1 = 4λ2
λ1 = λ2/2
λ1 = λ2/2
Which one of the following statements is not true?
Concentration of DO below 6 ppm is good for the growth of fish
Clean water would have a BOD value of less than 5 ppm
Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant
Oxides of sulphur, nitrogen and carbon are the most widespread air pollutant
If n =6, the correct sequence for filling of electrons will be
ns → (n-1) d → ( n - 2)f → np
ns - (n-2)f → np → (n-1)d
ns - np → (n-1)d → (n-2)f
ns - np → (n-1)d → (n-2)f
