 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following soap/detergent is least, reduce space biodegradable?
CH3 - (CH2)11 - OSO2Na
C17H35 - COONa
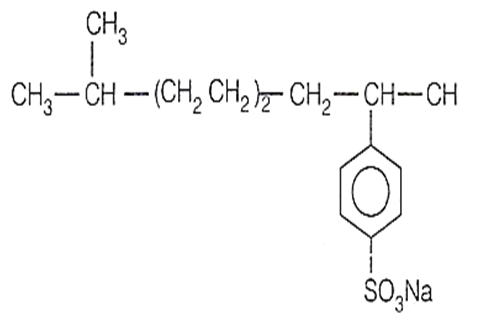
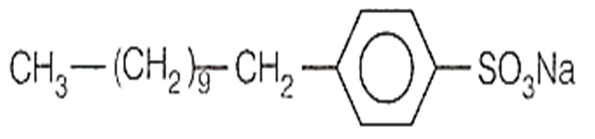
Buna-N, a synthetic rubber is copolymer of
H2C = CH - CH = CH2 and H5C6 - CH = CH2
H2C = CH - CN and H2C = CH - CH = CH2
H2C = CH - CCl = CH2 and H2C = CH - CH = CH2
H2C = CN - CN and H2C = CH - CCl = CH2
When a mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous NaHCO3 solution, then which of the following is right distribution?
| In ether | In sodium bicarbonate solution |
| Sodium hexanoate | 1- hexanol |
| 1- hexanol | Hexanoic acid |
| 1- hexanol | Sodium hexanoate |
| Hexanoic acid | 1- hexanol |
In the reaction
C7H8 X Y Z
Z is
o-bromotoluene
m-bromotoluene
p-bromotoluene
3-bromo-2, 2, 6- trichlorotoluene
The molal freezing point depression constant for benzene (C6H6) is 4.90 K Kg mol-1. Selenium exists as a polymer of the type Sex. When 3.26 g of selenium is dissolved in 226 g of benzene, the observed freezing point is 0.112C lower than that of pure benzene. The molecular formula of selenium is (atomic mass of Se = 78.8 g mol-1).
Se8
Se6
Se4
Se2
A.
Se8
To calculate the molar mass of Selenium (Se)
Mass of Selenium (WB) = 3.26 gm
Mass of Benzene (WA) = 226 gm = 0.226 kg
Depression in freezing point () = 0.112C
Molar depression constant (Kf) = 4.9K kg mol-1
Given, gram atomic mass of selenium = 78.8 g/mol
For Sex (78.8 g mol-1) = 631.08 g mol-1
or,
Thus, molecular formula of selenium = Se8
