 Multiple Choice Questions
Multiple Choice QuestionsA scientist proposes a new temperature scale in which the ice point is 25 X (X is the new unit of temperature) and the steam point is 305 X. The specific heat capacity of water in this new scale is (in J kg-1X-1)
4.2 × 103
3.0 × 103
1.2 × 103
1.5 × 103
One mole of a van der Waals' gas obeying the equation
undergoes the quasi-static cyclic process which is shown in the p-V diagram. The net heat absorbed by the gas in this process is
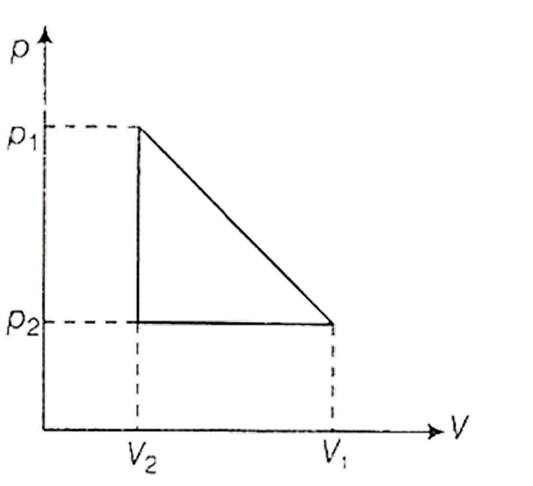
A.
For the cyclic process

A metal rod is fixed rigidly at two ends so as to prevent its thermal expansion. If L, α and Y respectively denote the length of the rod, coefficient of linear thermal expansion and Young's modulus of its material, then for an increase in temperature of the rod by ΔT, the longitudinal stress developed in the rod is
inversely proportional to α
inversely proportional to Y
directly proportional to ΔT/Y
independent of L
A drop of some liquid of volume 0.04 cm3 is placed on the surface of a glass slide. Then another glass slide is placed on it in such a way that the liquid forms a thin layer of area 20 cm2 between the surfaces of the two slides. To separate the slides a force of 16 x105 dyne has to be applied normal to the surfaces. The surface tension of the liquid is (in dyne-cm-1)
60
70
80
90
The displacement of a particle in a periodic motion is given by . This displacement may be considered as the result of superposition of n independent harmonic oscillations. Here n is
1
2
3
4
Same quantity of ice is filled in each of the two metal containers P and Q having the same size, shape and wall thickness but made of different materials. The containers are kept in identical surroundings. The ice in P melts completely in time t, whereas in Q takes a time t2. The ratio of thermal conductivities of the materials of P and Q is
t2 : t1
t1 : t2
t12 : t22
t22 : t12
A car is moving with a speed of 72 km-h-1 towards a roadside source that emits sound at a frequency of 850 Hz. The car driver listens to the sound while approaching the source and again while moving away from the source after crossing it. If the velocity ofsound is 340 ms-1, the difference of the two frequencies, the driver hears is
50 Hz
85 Hz
100 Hz
150 Hz
Sound waves are passing through two routes-one in straight path and the other along a semicircular path of radius r and are again combined into one pipe and superposed as shown in the figure. If the velocity of sound waves in the pipe is v, then frequencies of resultant waves of maximum amplitude will be integral multiples of

To determine the composition of a bimetallic alloy, a sample is first weight in air and then in water. These weights are found to be w1 and w2 respectively. If the densities of the two constituents metals are ρ1 and ρ2 respectively, then the weight of the first metal in the sample is (where ρw is the density of water)
A 10 W electric heater is used to heat a container filled with 0.5 kg of water. It is found that the temperature of water and the container rises by 3°K in 15 min. The container is then emptied, dried and filled with 2 kg of oil. The same heater now raises the temperature of container-oil system by 2°K in 20 min. Assuming that there is no heat loss in the process and the specific heat of water is 4200 J kg-1 K-1, the specific heat of oil in the same unit is equal to
1.50 × 103
2.55 × 103
3.00 × 103
5.10 × 103
