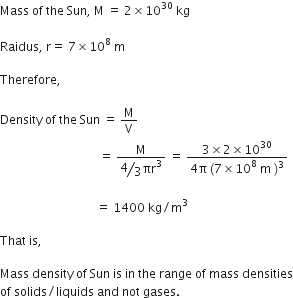Total time for which the two watches run, T = 100 years
Diffrence in the time = 0.02 sec
Error in 1 sec = 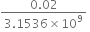 =
= 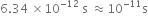
Thus, accuracy of 1 part is 1011.
Just as precise measurements are necessary in science, it is equally important to be able to make rough estimates of quantities using rudimentary ideas and common observations. Think of ways by which you can estimate the following (where an estimate is difficult to obtain, try to get an upper bound on the quantity):
(e) the number of air molecules in your classroom.
Let, the volume of the classroom be V.
One mole of air at NTP occupies 22.4 l.
i.e., 22.4 × 10–3 m3volume.
Number of molecules in one mole = 6.023 × 1023
∴ Number of molecules in room of volume V,
= 6.023 × 1023 × V / 22.4 × 10-3
= 134.915 × 1026 V
= 1.35 × 1028 V