During an isothermal expansion, a confined ideal gas does - 150 J of work against its surroundings. This implies that
300 J of heat has been added to the gas
no heat is transferred because the process is isothermal
150 J of heat has been added to the gas
150 J of heat has been added to the gas
C.
150 J of heat has been added to the gas
From the first law of thermodynamics
ΔU = Q + W
For isothermal process, ΔU = 0
therefore, Q = - W
Given W = - 150
Therefore, Q = + 150
When Q is positive, the heat is added to the gas.
When 1 kg of ice at 0oC melts to water 0oC, the resulting change in its entropy, taking latent heat of ice to be 80 cal/oC, is
8 x 104 cal /K
80 cal/K
293 cal/ K
293 cal/ K
C.
293 cal/ K
Change in entropy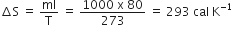
A mass of diatomic gas 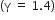 at a pressure of 2 atm is compressed adiabatically so that its temperature rise from 27oC to 927o C. The pressure of the gas is final state is
at a pressure of 2 atm is compressed adiabatically so that its temperature rise from 27oC to 927o C. The pressure of the gas is final state is
28 atm
68.7 atm
256 atm
256 atm
C.
256 atm
T1 = 273 + 27 = 300k
T2 = 273+927 = 1200 K
Gas equation for adiabatic process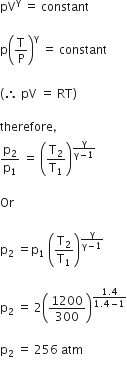
4.0 g of a gas occupies 22.4 L at NTP. The specific heat capacity of the gas at constant volume is 5.0 JK-1 mol-1. If the speed of sound in this gas at NTP is 952 ms-1, then the heat capacity at constant pressure is.
(Take gas constant R = 8.3 Jk-1 mol-1)
8.0 JK-1mol-1
7.5 JK-1mol-1
7.0 JK-1mol-1
7.0 JK-1mol-1
A.
8.0 JK-1mol-1
Given, M = 4 gm
V= 22.4 L,
CV = 5 JK-1 mol-1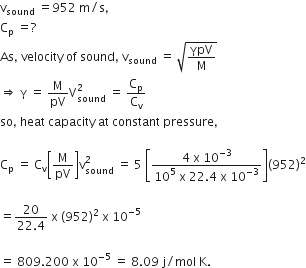
An ideal gas is compressed to half its initial volume by means of several process. Which of the process results in the maximum work done on the gas?
Adiabatic
Isobaric
Isochoric
Isochoric
A.
Adiabatic
Given, ideal gas is compressed to half its initial volume i.e.,
Vo = V/2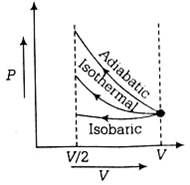
The isochoric process is one in which volume is kept constant, meaning that work done by the system will be zero ie.e Wisochoric = 0
As we know, work done on the gas = Area under curve i.e,
Wadiabatic > Wisothrmal > Wisochoric
