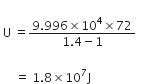Consider one mole of an ideal gas enclosed in a cylinder fitted with movable frictionless piston.
Let the gas be heated at constant volume first. Let the temperature of the gas increase by dT when dQ quantity of heat is supplied.
= dU + PdV
= dU ![]()
![]()
By first law of thermodynamics,
dQ = dU + dW = dU + PdV
or CpdT = Cv x dT + RdT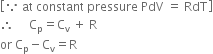
where Cp, Cv and R are in same units.
Let the latent heat of vaporisation of a liquid of mass m at its boiling temperature be L.
Let Vℓ be the volume of liquid and Vv be the volume of vapours and let evaporation take place at constant pressure P.
According to the first law of thermodynamics,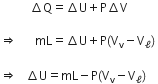
This is the required expression for change in internal energy.
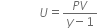 ... (1)
... (1)