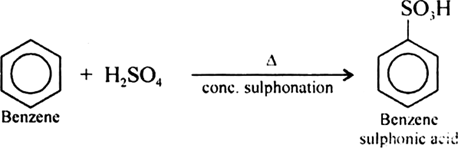
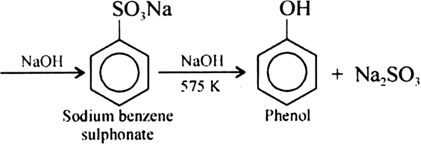
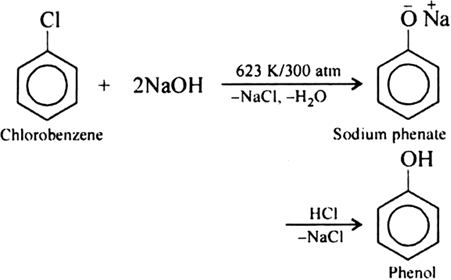
Write chemical reaction of preparation of phenol from chlorobenzene.
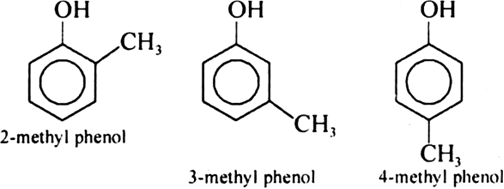
Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
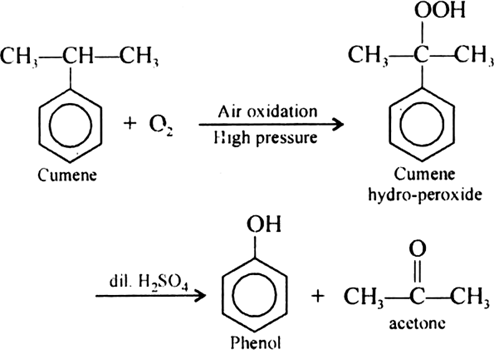
Give the equations of reaction for the preparation of phenol from cumene.
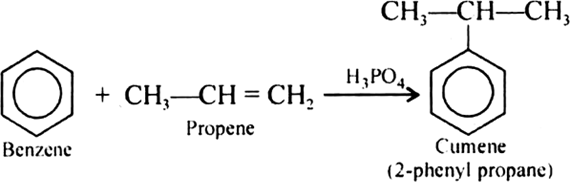
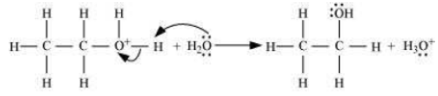
Write the mechanism of hydration of ethene to yield ethanol.
The mechanism of the reaction involves the following three step:
Step 1: Proptonation of ethene to form carbocation by electrophilic attack of H3O+.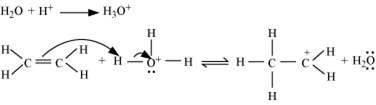
Step 2: Nucleophilic attack of water on carbocation.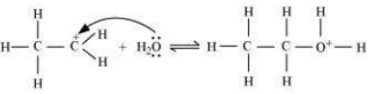
Step 3: Deprotonation to form an ethanol.