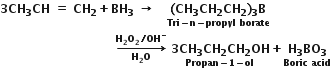(a) Eight isomeric alcohols are possible: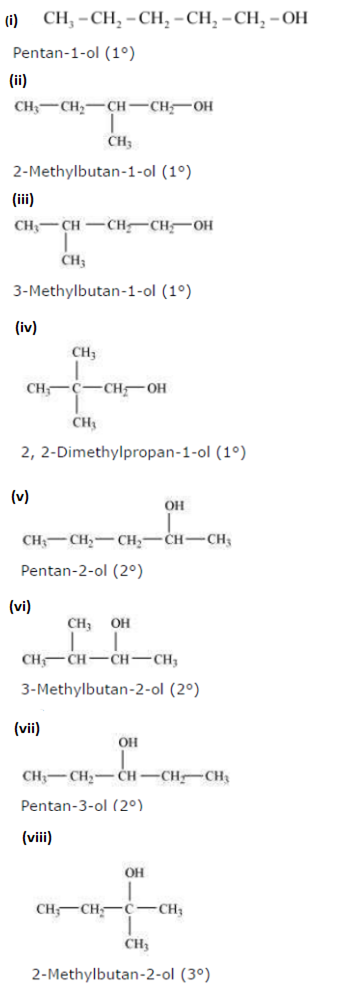
b) Primary alcohol: pentan-1-ol; methylbutan-1-ol; 3-methyl-1-ol; 2,2Dimethylpropan-1-ol.
Secondary alcohol: Pentan-2-ol; 3-methylbutan-2-ol; pentan-3-ol
Tertiary alcohol: 2-methylbutan-2-ol
The addition of borane followed by oxidation is known as the hydroboration-oxidation reaction.
In the first step, alkene reacts with diborane (B2H6) as boron hydride (BH3) to form an alkyl borane. In the next step, the alkyl borane is oxidised by alkaline H2O2 to form an alcohol. The indirect hydration proceeds according to Anti Markovnikoff’s rule. For example,