Explain why phenol has a much lower pKa value than ethanol.
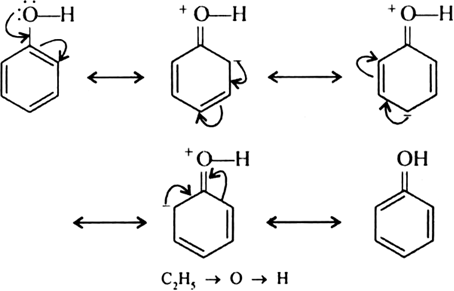
In phenol, the lone pair of oxygen participates into resonance with the benzene ring.
As a result, oxygen acquires a partial positive charge. The electron density of O—H bond then shifts towards oxygen decreases around H-atom. H-atom, therefore, can easily be removed as H+ ion. While in ethanol, ethyl group has a + I effect and increases the electron density around H of O—H group making it difficult to remove H as H+. Hence, phenol is more acidic than ethanol and has lower pKa value than ethanol.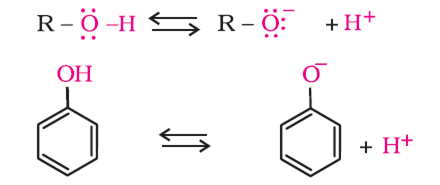
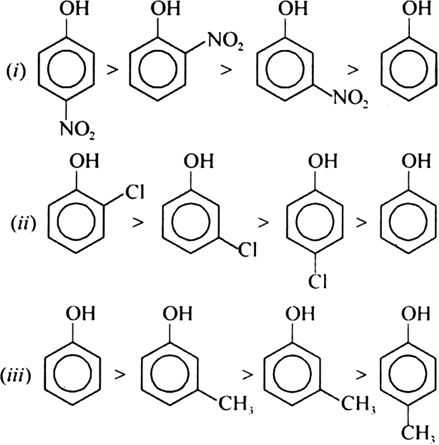
Arrrange the following groups of compounds in order of decreasing acidity: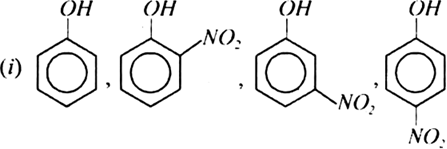
(ii) Phenol, o-chlorophenol, m-chlorophenol, p-chlorophenol.
(iii) o-cresol, m-cresol, p-cresol, phenol.
Ortho nitrophenol is more acidic than ortho-methoxyphenol. This is because the nitro-group is an electron-withdrawing group. The presence of this group in the ortho position in ortho nitro phenol decreases the electron density in the O−H bond. As a result, it is easier to lose a proton.Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
In the case of ortho methoxyphenol; methoxy group is an electron-releasing group. Thus, it increases the electron density in the O−H bond and hence, the proton cannot be given out easily. Hence, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
