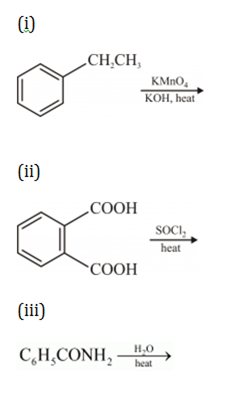
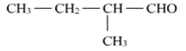Give chemical tests to distinguish between
(i) Propanal and propanone,
(ii) Benzaldehyde and acetophenone.
(b) How would you obtain
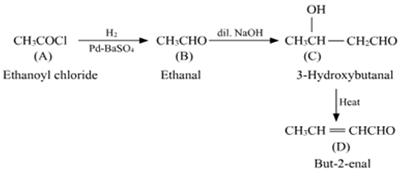
(i) But-2-enal from ethanal,
(ii) Butanoic acid from butanol,
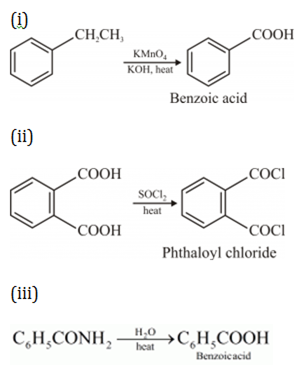
(iii) Benzoic acid from ethylbenzene?
(i) Propanal (CH3CH2CHO) can be distinguished from propanone (CH3COCH3) by iodoform test.
Being a methyl ketone, propanone on treatment with I2/NaOHundergoes iodoform reaction to give a yellow ppt. of iodoform ![]()
(ii) Benzaldehyde (C6H5CHO) and acetophenone (C6H5COCH3) can be distinguished by iodoform test.
Acetophenone, being a methyl ketone on treatment with I2 /NaOH undergoes iodoform reaction to give a yellow ppt. of iodoform. On the other hand, benzaldehyde does not give this test.
b)
i) 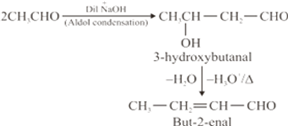
ii) ![]()
iii)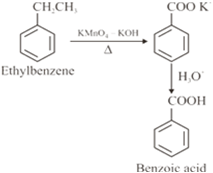
How will you bring about the following conversions?
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal
(i) Conversion of Propanone to Propane:
(ii) Conversion of Benzoyl chloride to benzaldehyde: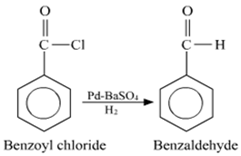
(iii) On treatment with dilute alkali, ethanol produces 3-hydroxybutanal gives But-2-enal on hheating.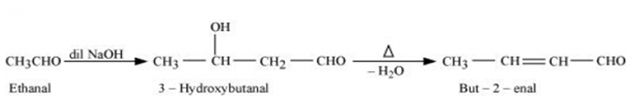
(a) Describe the following giving linked chemical equations:
(i) Cannizzaro reaction
(ii) Decarboxylation
(b) Complete the following chemical equations:
(i) Cannizaro reaction
In this reaction, the aldehydes which do not have a -hydrogen atom, undergo self-oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali.
Example:

(ii) Decarboxylation
The decarboxylation reaction can be carried out either by using soda lime or by electrolysis
Using soda lime: Sodium salts of carboxylic acids when heated with soda lime (NaOH + CaO) in the ratio 3:1) undergo decarboxylation reaction to yield alkanes.
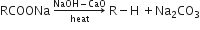
Electrolytic decarboxylation: Electrolysis of aqueous solutions of sodium or potassium salts of carboxylic acids give alkanes having twice the number of carbon atoms present in the alkyl group of acid.
This is known as Kolbe’s decarboxylation.
2RCOONa--> 2RCOO- + 2Na+
H2O-->2OH- + 2H+
At Anode:-
2RCOO- - 2e---> CO2 + R - R
At Cathode:-
2H+ + 2e----> H2
b)
2-chloro-2-methylpentane on reaction with sodium methoxide in methanol yields:
Both a and c
Only c
Both a and b
Both a and b
D.
Both a and b
Strong nucleophile  polar solvent (MeOH) gives elimination products Products over substitution products but all products are possible in different yields.
polar solvent (MeOH) gives elimination products Products over substitution products but all products are possible in different yields.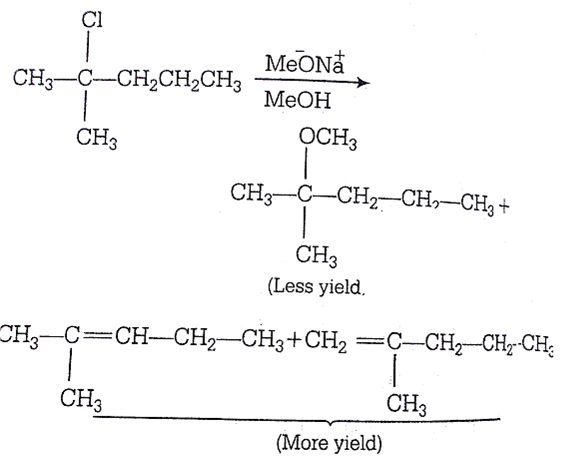
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions:
![]()
(b) Distinguish between the following:
(i) C6H5-COCH3 and C6H5 - CHO
(ii) Benzoic acid and methyl benzoate
(c) Write the structure of 2- methylbutanal.
(b)
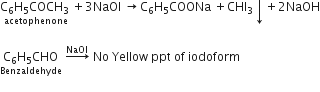
(i) Acetophenone has methyl group attached to carbonyl carbon while benzaldehyde does not. Therefore, we can use iodoform test to distinguish between the two. Acetophenone will undergo iodoform test and give a yellow precipitate.
C6H5-COCH3 ![]() C6H5COOH + CHI3
C6H5COOH + CHI3
Acetophenone (yellow ppt.)
C6H5CHO ![]() No reaction
No reaction
Benzaldehyde
(ii) Benzoic acid can react with sodium bicarbonate to give brisk effervescence due to the release of CO2, while methyl benzoate does not.
C6H5COOH + NaHCO3----> C6H5COONa + H2O + CO2
Benzoic acid (brisk effervescence)
C6H5COOCH3 + NaHCO3---> No reaction
Methyl benzoate
(c) The Structure of 2- methylbutanal is: