
|
exp.
|
[A]/ |
[B]/M |
Initial rate of formation |
|
I |
0.1 |
0.1 |
|
|
II |
- |
0.2 |
4.0 x 10–2
|
|
III |
0.4 |
0.4 |
- |
|
IV |
- |
0.2 |
2.0 x 10–2 |
Rate law for the reaction is given by:
Rate = k [A]1 [B]0 = k[A]
2.0 x 10–2 mol L–1 min–1 = k[0.1 mol L–1]
Rate constant,
Rate constant = k = 0.2 min–1.
(i) In experiment II Rate = k[A]
(ii) In experiment III
Rate = k[A]
= 0.2 min–1 x 0.4 M
= 0.08 min–1
= 8.0 x 10–2 M min–1
(iii) In experiment IV
Rate = k[A]
Thus the completed table is
|
exp.
|
[A]/ |
[B]/M |
Initial rate of formation |
|
I |
0.1 |
0.1 |
|
|
II |
0.2 |
0.2 |
4.0 x 10–2
|
|
III |
0.4 |
0.4 |
8.0 x 10-2 |
|
IV |
0.1 |
0.2 |
2.0 x 10–2 |
The rate constant of reaction increases with increase of temperature. This increase is generally two fold to five fold for 10 K rise in temperature. This is explained on the basis of collision theory. The main parts of collision theory are as follows:
(i) For a reaction to occur, there must be collision between the reacting species.
(ii) Only a certain fraction of total collisions are effective in forming the products.
(iii) For effective collisions, the molecule must possess the sufficient energy (equal or greater
than threshold energy) as well as proper orientation.
On the basis above conclusions, the rate of reaction is given by
Rate =f x 2 (where f is the effective collisions and is total number of collisions per unit volume per second).
Quantitatively, the effect of temperature on the rate of a reaction and hence on the rate constant k was proposed by Arrhenius. The equation, called Arrhenius equation is usually written in the form
...(i)
.
where A is a constant called frequency factor (because it gives the frequency of binary collisions of the reacting molecules per second per litre, E0 is the energy of activation, R is a gas constant and T is the absolute temperature. The factor e–Ea/RT gives the fraction of molecules having energy equal to or greater than the activation energy, Ea.
The energy of activation (Ea) is an important quantity and it is characteristic of the reaction. Using the above equation, its value can be calculated.
Taking logarithm or both sides of equation (i), we get,
If the value of the rate constant at temper-atures T1 and T2 are k1 and k2 respectively, then we have
Subtracting eqn. (i) from eqn. (ii), we get
or
Thus knowing the values of the constant k1 and k2 at two different temperature T1 and T2, the value of Ea can be calculated.
Rate constant of reaction,
k = 60 S–1
t15/16 = ?
Rate constant of first order reaction is given by.
where 15 / 16th the reaction is over the
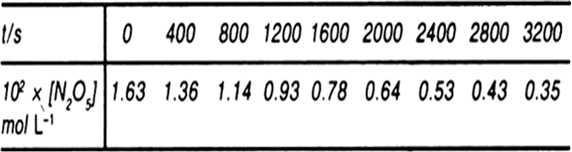
(a) Plot [N2O5] again t.
(b) Find the half life period for the reaction.
(c) Draw a graph between log [N2O5] and t.
(d) What is rate law?
(e) Calculate the rate constant.
(f) Calculate the half life period from K and compare it with (ii).
![(a) Plot of [N2O5] vs. time.
(b) Time taken for the concentration of](/application/zrc/images/qvar/CHEN12046780-1.png)
(b) Time taken for the concentration of N2O5 to change from 1.63 x 10–2 mol L–1 to half the value.
t0.5 = 1420 s [from the graph] (c) Plot of log (N2O5) vs. time.
|
t |
log10[N2O5] |
|
0 400 800 1200 1600 2000 2400 2800 3200 3600 |
- 1.7918 - 1.8665 - 1.9431 - 2.0315 - 2.1079 - 2.1938 - 2.2757 - 2.3752 - 2.4559 - 2.5376 |
![(a) Plot of [N2O5] vs. time.
(b) Time taken for the concentration of](/application/zrc/images/qvar/CHEN12046780-2.png)
![(a) Plot of [N2O5] vs. time.
(b) Time taken for the concentration of](/application/zrc/images/qvar/CHEN12046780-3.png)
