 Multiple Choice Questions
Multiple Choice QuestionsThe volume of a colloidal particle, VC as compared to the volume of a solute particle in a true solution VS, could be
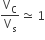
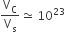
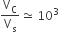
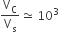
C.
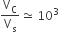
The solubility product of a salt having general formula MX2, in water is 4 x 10-12. The concentration of M2+ ions in the aqueous solution of the salt is
2.0 x 10-6 M
1.0 x 10-4 M
1.6 x 10-4 M
1.6 x 10-4 M
Benzene and toluene form nearly ideal solutions. At 20o C, the vapour pressure of benzene is 75 torr and that of toluene is 22 torr. The partial vapour pressure of benzene at 20o C for a solution containing 78 g of benzene and 46 g of toluene in torr is
50
25
37.5
37.5
Two solutions of a substance (non-electrolyte) are mixed in the following manner. 480 ml of 1.5 M first solution + 520 mL of 1.2 M second solution. What is the molarity of the final mixture?
1.20 M
1.50 M
1.344 M
1.344 M
A reaction involving two different reactants can never be
Unimolecular reaction
First order reaction
second order reaction
second order reaction
During the process of electrolytic refining of copper, some metals present as impurity settle as ‘anode mud’ These are
Sn and Ag
Pb and Zn
Ag and Au
Ag and Au
| Electrolyte | KCl | KNO3 | HCl | NaOAc | NaCl |
| ∧∞S cm2 mol- | 149.9 | 145.0 | 426.2 | 91.0 | 126.5 |
517.2
552.7
390.7
390.7
A schematic plot of In Keq versus inverse of temperature for a reaction is shown below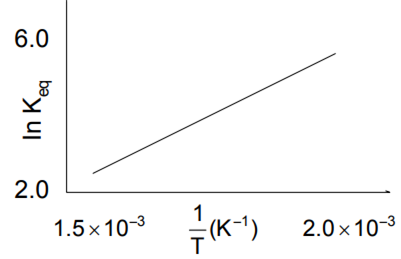
The reaction must be
exothermic
endothermic
one with negligible enthalpy change temperature
one with negligible enthalpy change temperature
The disperse phase in colloidal iron (III) hydroxide and colloidal gold is positively and negatively charged, respectively, which of the following statements is NOT correct?
magnesium chloride solution coagulates, the gold sol more readily than the iron (III) hydroxide sol.
sodium sulphate solution causes coagulation in both sols
mixing the sols has no effect
mixing the sols has no effect
Based on lattice energy and other considerations which one of the following alkali metal chlorides is expected to have the highest melting point.
LiCl
NaCl
KCl
KCl
