 Multiple Choice Questions
Multiple Choice QuestionsThe cell, Zn|Zn2+ (1M)|| Cu2+ (1M|Cu(Eocell = 1.10 V), was allowed to be completely discharged at 298 K. THe relative concentration of Zn2+ to Cu2+ 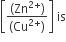
antilog (24.08)
37.3
1037.3
1037.3
C.
1037.3
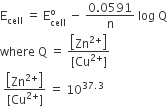
Consider the reaction, 2A + B → Products. When the concentration of B alone was doubled, the half-life did not change. When the concentration of An alone was doubled, the rate increased by two times. The unit of rate constant for this reaction is –
L mol–1 s–1
no unit
mol L–1s–1
mol L–1s–1
Identify the incorrect statement among the following –
d-Block elements show irregular and erratic chemical properties among themselves
La and Lu have partially filled d orbitals and no other partially filled orbitals
The chemistry of various lanthanoids is very similar
The chemistry of various lanthanoids is very similar
Which one of the following has a square planar geometry?
[CoCl4]2-
[FeCl4]2–
[NiCl4] 2–
[NiCl4] 2–
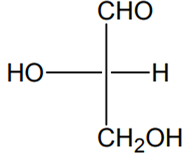
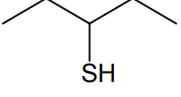
Which of the following molecules is expected to rotate the plane of plane-polarised light?


In which of the following ionization processes, the bond order has increased and the magnetic behaviour has changed?
C2 → C2+
NO → NO+
O2 → O2+
O2 → O2+
The actinoids exhibits more number of oxidation states in general than the lanthanoids. This is because
the 5f orbitals are more buried than the 4f orbitals
there is a similarity between 4f and 5f orbitals in their angular part of the wave function
the actinoids are more reactive than the lanthanoids
the actinoids are more reactive than the lanthanoids
Equal masses of methane and oxygen are mixed in an empty container at 25ºC. The fraction of the total pressure exerted by oxygen is
2/3
(1/3) x (273 / 298)
1/3
1/3
A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol–1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 gcm–3, molar mass of the substance will be –
90.0g mol–1
115.0g mol–1
105.0g mol–1
105.0g mol–1
In a saturated solution of the sparingly soluble strong electrolyte AgIO3 (molecular mass = 283) the equilibrium which sets in is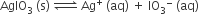
If the solubility product constant Ksp of AgIO3 at a given temperature is 1.0 × 10-8, what is the mass of AgIO3 contained in 100 ml of its saturated solution
28.3 × 10–2 g
2.83 × 10–3 g
1.0 × 10–7 g
1.0 × 10–7 g
