 Multiple Choice Questions
Multiple Choice QuestionsA radioactive element gets spilled over the floor of a room. Its half-life period is 30 days. If the initial activity is ten times the permissible value, after how many days will it be safe to enter the room ?
1000 days
300 days
50 days
50 days
D.
50 days
Which one of the following conformation of cyclohexane is chiral?
Twist boat
Rigid
chair
chair
Which of the following nuclear reactions will generate an isotope?
neutron particle emission
positron emission
α-particle emission
α-particle emission
The equivalent conductances of two strong electrolytes at infinite dilution in H2O (where ions move freely through a solution) at 25°C are given below: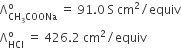
What additional information/quantity one needs to calculate Λ of an aqueous solution of acetic acid?
∧° of NaCl
Λº of CH3COOH
The limiting equivalent conductance of 
The limiting equivalent conductance of 
The stability of dihalides of Si, Ge, Sn and Pb increases steadily in the sequence
GeX2 << SiX2 << SnX2<< PbX2
SiX2 << GeX2 << PbX2<< SnX2
SiX2 << GeX2 << SnX2<< PbX2
SiX2 << GeX2 << SnX2<< PbX2
Identify the incorrect statement among the following
Ozone reacts with SO2 to give SO3
Silicon reacts with NaOH(aq) in the presence of air to give Na2SiO3 and H2O
Cl2 reacts with excess of NH3 to give N2 and HCl
Cl2 reacts with excess of NH3 to give N2 and HCl
The density (in g mL–1) of a 3.60 M sulphuric acid solution that is 29% H2SO4 (Molar mass = 98 g mol-) by mass will be
1.64
1.88
1.22
1.22
The first and second dissociation constants of an acid H2A are 1.0 × 10–5 & 5.0 × 10–10 respectively. The overall dissociation constant of the acid will be
5.0 × 10–5
5.0 × 1015
5.0 × 10–15
5.0 × 10–15
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be
350
300
360
360
