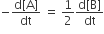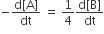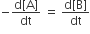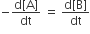Multiple Choice Questions
Multiple Choice QuestionsThe electrophile, E⊕ attacks the benzene ring to generate the intermediate σ-complex. Of the following, which σ-complex is of lowest energy?
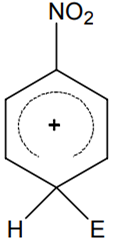
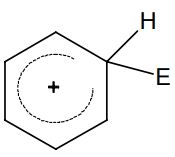
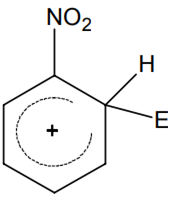

B.

-NO2 is electron withdrawing which will destabilize σ - complex.
Which one of the following is the correct statement?
Boric acid is a protonic acid
Beryllium exhibits coordination number of six
Chlorides of both beryllium and aluminium have bridged chloride structures in solid phase
Chlorides of both beryllium and aluminium have bridged chloride structures in solid phase
Amount of oxalic acid present in a solution can be determined by its titration with KMnO4 solution in the presence of H2SO4. The titration gives unsatisfactory result when carried out in the presence of HCl, because HCl
gets oxidised by oxalic acid to chlorine
furnishes H+ions in addition to those from oxalic acid
reduces permanganate to Mn2+
reduces permanganate to Mn2+
In context with the industrial preparation of hydrogen from water gas (CO + H2), which of the following is the correct statement?
CO and H2 are fractionally separated using differences in their densities
CO is removed by absorption in aqueous Cu2Cl2 solution
H2 is removed through occlusion with Pd
H2 is removed through occlusion with Pd
The correct decreasing order of priority for the functional groups of organic compounds in the IUPAC system of nomenclature is
−COOH, −SO3H, −CONH2, −CHO
−SO3H, −COOH, −CONH2, −CHO
−CHO, −COOH, −SO3H, −CONH2
−CHO, −COOH, −SO3H, −CONH2
The coordination number and the oxidation state of the element ‘E’ in the complex [E(en)2(C2O4)]NO2 (where(en) is ethylene diamine) are, respectively,
6 and 2
4 and 2
4 and 3
4 and 3
Larger number of oxidation states are exhibited by the actinoids than those by the lanthanoids, the main reason being
4f orbitals more diffused than the 5f orbitals
lesser energy difference between 5f and 6d than between 4f and 5d orbitals
more energy difference between 5f and 6d than between 4f and 5d orbitals
more energy difference between 5f and 6d than between 4f and 5d orbitals
In which of the following octahedral complexes of Co (at. no. 27), will the magnitude of ∆o be the highest?
[Co(CN)6]3−
[Co(C2O4)3]3−
[Co(H2O)6]3+
[Co(H2O)6]3+
At 80o C, the vapour pressure of pure liquid ‘A’ is 520 mm Hg and that of pure liquid ‘B’ is 1000 mm Hg. If a mixture solution of ‘A’ and ‘B’ boils at 80o C and 1 atm pressure, the amount of ‘A’ in the mixture is (1 atm = 760 mm Hg)
52 mol percent
34 mol percent
48 mol percent
48 mol percent
For a reaction 1/2A →2B, rate of disappearance of ‘A’ is related to the rate of appearance of ‘B’ by the expression