 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following factors is of no significance for roasting sulphide ores to the oxides and not subjecting the sulphide ores to carbon reduction directly?
Metal sulphides are thermodynamically more stable than CS2
CO2 is thermodynamically more stable than CS2
Metal sulphides are less stable than the corresponding oxides
Metal sulphides are less stable than the corresponding oxides
A.
Metal sulphides are thermodynamically more stable than CS2
The vapour pressure of water at 20oC is 17.5 mm Hg. If 18 g of glucose (C6H12O6) is added to 178.2 g of water at 20oC, the vapour pressure of the resulting solution will be
17.675 mm Hg
15.750 mm Hg
16.500 mm Hg
16.500 mm Hg
In a compound atoms of element Y from ccp lattice and those of element X occupy 2/3rd of tetrahedral voids. The formula of the compound will be
X4Y3
X2Y3
X2Y
X2Y
Gold numbers of protective colloids A, B, C and D are 0.50, 0.01, 0.10 and 0.005, respectively. The correct order of their protective powers is
D < A < C < B
C < B < D < A
A < C < B < D
A < C < B < D
Toluene is nitrated and the resulting product is reduced with tin and hydrochloric acid. The product so obtained is diazotised and then heated with cuprous bromide. The reaction mixture so formed contains
mixture of o− and p−bromotoluenes
mixture of o− and p−dibromobenzenes
mixture of o− and p−bromoanilines
mixture of o− and p−bromoanilines
The organic chloro compound, which shows complete stereochemical inversion during a SN2 reaction,is
(C2H5)2CHCl
(CH3)3CCl
(CH3)2CHCl
(CH3)2CHCl
Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid,gives
2,4,6-trinitrobenzene
o-nitrophenol
p-nitrophenol
p-nitrophenol
In the following sequence of reactions, the alkene affords the compound ‘B’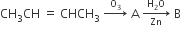
The compound B is
CH3CH2CHO
CH3COCH3
CH3CH2COCH3
CH3CH2COCH3
