 Multiple Choice Questions
Multiple Choice QuestionsVery pure hydrogen (99.9%) can be made by which of the following processes?
Mixing natural hydrocarbons of high molecular weight
Electrolysis of water
Electrolysis of water
C.
Electrolysis of water
Which branched chain isomer of the hydrocarbon with molecular mass 72u gives only one isomer of mono substituted alkyl halide?
Tertiary butyl chloride
Neopentane
Isohexane
Isohexane
Which among the following will be named as dibromidobis(ethylene diamine)chromium(III) bromide?
[Cr(en)3]Br3
[Cr(en)2Br2]Br
[Cr(en)Br4]-
[Cr(en)Br4]-
Which method of purification is represented by the following equation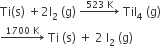
zone refining
cupellation
Polling
Polling
Lithium forms body centred cubic structure. The length of the side of its unit cell is 351 pm. Atomic radius of the lithium will be
75 pm
300 pm
240 pm
240 pm
The density of a solution prepared by dissolving 120 g of urea (mol. Mass = 60 u ) in 1000g of water is 1.15 g/mL. The molarity of this solution is
0.50 M
1.78 M
1.02 M
1.02 M
Which one of the following statements is correct?
All amino acids except lysine are optically active
All amino acids are optically active
All amino acids except glycine are optically active
All amino acids except glycine are optically active
How many chiral compounds are possible on monochlorination of 2–methyl butane?
8
2
4
4
