 Multiple Choice Questions
Multiple Choice QuestionsCsCl crystallises in body centred cubic lattice. If 'a' its edge length, then which of the following expression is correct?

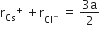
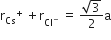
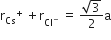
C.
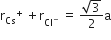
In CsCl, Cl- lie at corners of simple cube and Cs+ at the body centre, Hence, along the body diagonal, Cs+ and Cl- touch each other so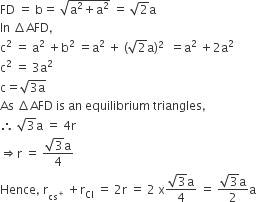
Consider separate solution of 0.500 M C2H5OH (aq), 0.100 M Mg3(PO4)2 (aq) 0.250 M KBr (aq) and 0.125 M Na3PO4 (aq) at 25oC. Which statement is true about these solutions, assuming all salts to be strong electrolytes?
They all have the same osmotic pressure
0.100 M Mg3(PO4)2 (aq) has the highest osmotic pressure
0.125 M Na3PO (aq) has the highest osmotic pressure
0.125 M Na3PO (aq) has the highest osmotic pressure
For the non- stoichiometric reaction 2A + B → C + D, the following kinetic data were obtained in three separate experiment, all at 298 K.
| Initial concentration (A) | Initial concnetration (B) | Initial rate of formation of C (mol L-1 S-1) | |
| 1 | 0.1 M | 0.1 M | 1.2 x 10-3 |
| 2 | 0.1 M | 0.2 M | 1.2 x 10-3 |
| 3 | 0.2 M | 0.1 M | 2.4 x 10-3 |
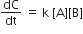
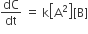
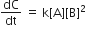
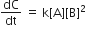
Among the following oxoacids, the correct decreasing order of acid strength is
HOCl > HClO2>HClO3 > HClO4
HClO4>HOCl> HClO2>HClO3
HClO4>HClO3>HClO2>HOCl
HClO4>HClO3>HClO2>HOCl
The metal that cannot be obtained by electrolysis of an aqueous solution of its salts is
Ag
Ca
Cu
Cu
The octahedral complex of a metal ion M3+ with four Monodentate Ligands, L1, L2, L3 and L4 absorb wavelengths in the region of red, green, yellow and blue respectively. The increasing order of ligand strength of the four ligands is
L4< L3<L2<L1
L1<L3<L2<L4
L3<L2<L4<L1
L3<L2<L4<L1
Which of the following properties are not shown by NO?
It is diamagnetic in gaseous state
It is a neutral oxide
It combines with oxygen to form nitrogen dioxide
It combines with oxygen to form nitrogen dioxide
The ratio of masses of oxygen and nitrogen of a particular gaseous mixture is 1:4. The ratio of number of their molecule is
1:4
7:32
1:8
1:8
Given below are the half-cell reactions
Mn2+ + 2e- → Mn; Eo = - 1.18 eV
2(Mn3+ + e- →Mn2+); Eo = +1.51 eV
The Eo for 3Mn2+ → Mn + 2Mn3+ will be
-2.69 V; the reaction will not occur
-2.69 V; the reaction will occure
-0.33 V; the reaction will not occur
-0.33 V; the reaction will not occur
Which series of reactions correctly represents chemical relations related to iron and its compound?




