 Multiple Choice Questions
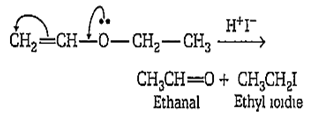
Multiple Choice QuestionsWhen CH2=CH-O-CH2-CH3 reacts with one mole of HI, one of the products formed is
ethane
ethanol
iodoethene
ethanal
D.
ethanal
Allyl ethyl ether on reaction with HI produces ethanal and ethyl iodide by means of necleophilic substitution reaction as shown below:
0.44 g of a monohydric alcohol when added to methylmagnesium iodide in ether liberates at STP, 112 cm3 of methane. With PCC the same alcohol forms a carbonyl compound that answers silver mirror test. The monohydric alcohol is
H3C-CH(OH)-CH2-CH3
(CH3)3C-CH2OH
H3C-CH(OH)-CH2-CH2-CH3
(CH3)2CH-CH2OH
An incorrect statement with respect to SN1 and SN2 mechanisms for alkyl halide is
A strong nucleophile in an aprotic solvent increases the rate or favours SN2 reaction.
Competing reaction for an SN2 reaction is rearrangement.
SN1 reactions can be catalysed by some Lewis acids.
A weak nucleophile and a protic solvent increases the rate or favours SN1 reaction.
Butylated hydroxyl toluene as a food additive acts as
antioxidant
flavouring agent
colouring agnet
emulsifier
Iodoform reaction is answered by all, except
CH3-CH(OH)-CH2-COOH
CH3CHO
CH3-CH2-OH
CH3-CH2-CH2OH
The statement that is not correct is
Aldose or ketose sugars in alkailne medium do not isomerise
Carbohydrates are optically active
Pentaacetate of glucose does not react with hydroxylamine
Lactose has glycosidc linkage between C4 of glucose and C1 of galactose unit.
