 Multiple Choice Questions
Multiple Choice QuestionsWhen BaCl2 is added to an aqueous salt solution, a white precipitate is obtained. The anion among CO-, SO- and SO that was present in the solution can be
CO but not any of the other two
SO but not any of the other two
SO but not any of the other two
Any of them
D.
Any of them
If CO, SO and SO are present alongwith BaCl2, these can also show white precipitate (as precipitate of all these are also white).
In the IUPAC system, PhCH2CH2CO2H is named as
3-phenylpropanoic acid
benzylacetic acid
carboxyethylbenzene
2-phenylpropanoic acid
The isomerisation of 1-butyne to 2-butyne can be achieved by treatment with
hydrochloric acid
ammoniacal silver nitrate
ammoniacal cuprous chloride
ethanolic potassium hydroxide
The correct order of acid strengths of benzoic acid (X), peroxybenzoic acid (Y) and p-nitrobenzoic acid (Z) is
Y > Z > X
Z > Y > X
Z > X > Y
Y > X > Z
The reduction of benzenediazonium chloride to phenyl hydrazine can be accomplished by
SnCl2, HCl
Na2SO3
CH3CH2OH
H3PO2
The major product(s) obtained form the following reaction of 1 mole of hexadeuteriobenzene is/are
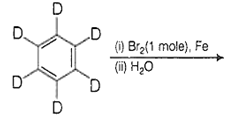
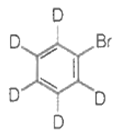
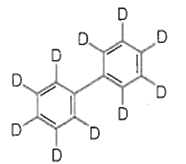
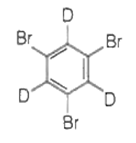
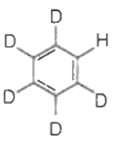
The conversion of CH3-CH2-COOH to
![]()
accomplished by
SOCl2, LiAlH4, ethylene glycol
SOCl2, KMnO4, NH2NH2
SnCl2, HCl, Na2SO3
HCl, SnCl2, ethylene glycol
The number of unpaired electrons in[NiCl4]2-, Ni(CO)4 and (Cu(NH3)4]2+ respectively are
0, 2, 1
2, 0, 1
0, 2, 1
2, 2, 0
