 Multiple Choice Questions
Multiple Choice QuestionsThe osmotic pressure of a dilute solution of an ionic compound XY in water is four times that of a solution of 0.01 M BaCl2 in water. Assuming complete dissociation of the given ionic compounds is water, the concentration of XY (in mol L-1 ) in solution is
4 × 10-4
16 × 10-4
4 × 10-2
6 × 10-2
D.
6 × 10-2
Aniline dissolved in dilute HCl is reacted with sodium nitrite at 0ºC. This solution was added dropwise to a solution containing equimolar mixture of aniline and phenol in dil. HCl. The structure of the major product is
![]()
![]()
![]()
![]()
The major product of the following reaction is
CH3C≡CH
CH3C (I) Cl CHD2
CH3CD (Cl) CHD (I)
CH3CD2CH(Cl) (I)
CH3CD(I)CHD (Cl)
The increasing order of reactivity of the following compounds towards aromatic electrophilic substitution reaction is
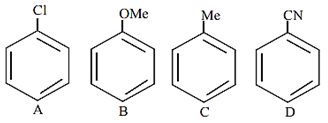
D < A < C < B
B < C < A < D
D < B < A< C
A < B < C < D
Which of the following statement is not true about sucrose?
On hydrolysis, it produces glucose and fructose
It is also named as invert sugar
It is non-reducing sugar
The glycosidic linkage is present between C1 of α-glucose and C1 of β- fructose.
The major product of the following reaction is
CH3CH=CHCO2CH3
CH3CH2CH2CO2CH3
CH3CH2CH2CH2OH
CH3CH2CH2CHO
CH3CH=CHCH2OH
